Introduction
Cisplatin is a simple inorganic molecule that commonly used to treat malignant tumors, especially testicular cancer [1]. Its overall cure rate exceeds 90%, and this figure rises almost 100% for early-stage of cancers. Cisplatin is a highly reactive molecule that binds to DNA and causes defects in DNA templates as well as DNA damage, but cisplatin treatment is limited by its severe side effects in normal tissues, especially in the kidney [2]. Cisplatin nephrotoxicity occurs in nearly 34% of cancer patients after 10 days of cisplatin treatment, and it is also seen as lower glomerular filtration and higher serum creatinine [3]. Kidney tubular cells take up cisplatin, which subsequently induces tubular cell death as a common histopathological feature of nephrotoxicity. During cisplatin nephrotoxicity in vivo, apoptosis and necrosis are simultaneously induced in kidney tubules; whereas in cultured kidney tubular epithelial cells in vitro, both cell death types are dependent on cisplatin concentration [456]. Most recent studies have focused on finding ways to inhibit apoptosis, but not necrosis, induced by cisplatin in kidney tubular cells [789]. Our previous study has demonstrated that the excessive activation of poly(ADP-ribose) polymerase 1 (PARP1) contributes to necrosis induced by cisplatin, and its inhibition efficaciously prevents cisplatin nephrotoxicity [5101112]. Since the inhibition of apoptosis and necrosis synergistically ameliorates cisplatin nephrotoxicity [13], finding more ways to inhibit necrosis is of critical importance to improve cancer chemotherapy.
Polyamines, an intrinsic constituent of all eukaryotic cells, are present in foods such as meats, green leafy plants and dairy products. Spermidine is a polyamine that is essential to nearly every cellular function, including DNA replication, transcription and translation [1415]. Furthermore, spermidine supplement inhibits necrosis in aged yeast cells, resulting in an increase in life span and improvement in health [16]. Recently, our previous studies have demonstrated that spermidine is protective against kidney ischemia and reperfusion injury [1718]. However, the anti-necrotic mechanism of exogenous spermidine on cisplatin-induced nephrotoxicity remains unknown. Therefore, we hypothesized that exogenous spermidine protects against cisplatin nephrotoxicity by inhibiting necrosis. We investigate this hypothesis by assessing whether spermidine decreases necrotic cell death in an in vivo cisplatin nephrotoxicity model, and if so, whether genetic inhibition of ornithine decarboxylase (ODC) enzyme that synthesizes spermidine accelerates cisplatin injury.
Materials and Methods
Animal preparation and cisplatin nephrotoxicity
Male C57BL/6 mice aged 8 to 10 weeks were purchased from Orient Bio (Seongnam, Korea). All mouse experiments were performed in accordance with the animal protocols approved by the Institutional Animal Care and Use Committee of Jeju National University. Mice were intraperitoneally injected with cisplatin (a single dose of 30 mg/kg body weight, R&D Systems, Minneapolis, MN, USA) to induce nephrotoxicity or with 0.9% saline (control). Spermidine (1 to 10 mg/kg body weight; Sigma, St. Louis, MO, USA) was dissolved in distilled water (vehicle) and was administered orally at 24 and 1 hour before cisplatin injection. To use a small interfering RNA (siRNA) targeting ODC in vivo, ODC siSTABLE siRNA (siODC, 50 µg in 1 ml phosphate buffered saline [PBS]) modified from siGENOME (Dharmacon, Lafayette, CA, USA) or siSTABLE non-targeting siRNA (siControl, 50 µg in 1 ml PBS) was injected within 10 seconds into tail veins at 48 and 24 hours before cisplatin injection, as previously described [1920]. When the mice were anesthetized, the kidneys were either fixed in 4% paraformaldehyde for histological studies or snap-frozen in liquid nitrogen for biochemical studies.
Spermidine quantification
Spermidine contents in kidney tissues and plasma were measured by Flexar FX-10 high-performance liquid chromatography (PerkinElmer, Waltham, MA, USA), as previously described [21]. Plasma was prepared by centrifugation of ethylenediaminetetraacetic acid-collected blood at 2,500 ×g for 20 minutes. Kidney samples were homogenized with 0.3 M perchloric acid and centrifuged at 4,000 ×g for 10 minutes. The supernatants were added to 10 nM internal standard (1,6-hexanediamine), derivatization with benzoyl chloride, and extracted with chloroform. The derivatives were separated on Hypersil ODS (C18) classical HPLC columns (5 µm, Thermo Fisher Scientific, Waltham, MA, USA). The column eluate was monitored by a Jenway 7305 spectrophotometer (Bibby Scientific Limited, Stone, UK) at 229 nm.
Tubular necrosis score
Histological tubular injury due to acute tubular necrosis in the cortex and outer medulla of periodic acid-Schiff (PAS)-stained kidney sections was scored by counting the percentage of tubules that displayed tubular necrosis (no nuclei, cast formation, or tubular dilation) as follows: 0, normal; 1, 1% to 10%; 2, 11% to 25%; 3, 26% to 50%; 4, 51% to 75%; and 5, 76 to 100%. Ten randomly chosen high-power (×200 magnification) fields per kidney were used for the scoring [1124].
Enzyme-linked immunosorbent assay
Lipid hydroperoxide, 8-hydroxy-2'-deoxyguanosine (8-OHdG), 8-nitroguanine, PARP1 activity and adenosine triphosphate (ATP) assays were performed with whole kidneys or cells using a lipid hydroperoxide assay kit (Cayman, Ann Arbor, MI, USA), a DNA/RNA oxidative damage ELISA kit (Cayman), a 8-nitroguanine DNA/RNA damage ELISA kit (Cell Biolabs, San Diego, CA, USA), a universal PARP colorimetric assay kit (Trevigen, Gaithersburg, MD, USA), and an ATP fluorometric assay kit (BioVision, Mountain View, CA, USA) according to the respective manufacturer's instructions [25].
Western blot
Electrophoresis of protein extracts obtained from whole kidneys using tris-glycine buffer systems and subsequent blotting were performed as previously described [2627]. The membranes were incubated with antibodies against ODC (Proteintech, Chicago, IL, USA), caspase-3 (Cell Signaling Technology, Beverly, MA, USA) and β-actin (Sigma). Peroxidase-conjugated secondary antibodies (Vector Laboratories, Burlingame, CA, USA) were applied. After that, a chemiluminescence reagent (PerkinElmer, Boston, MA, USA) was used to detect the proteins. Antibodies for β-actin were used as a loading control on stripped membranes. The bands were quantified using AzureSpot analysis software (Azure Biosystems, Dublin, CA, USA).
Statistical analysis
All data are expressed as means±standard error of mean. Analysis of variance was used to compare data among groups using Systat SigmaPlot (Systat Software Inc., San Jose, CA, USA). Differences between the two groups were assessed using two-tailed unpaired Student's t tests. P-values of <0.05 were considered statistically significant.
Results
Exogenous spermidine attenuates tubular necrosis and kidney dysfunction during cisplatin nephrotoxicity
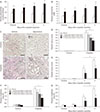
Cellular spermidine content in kidney tissue was significantly increased in mice treated with spermidine as compared to vehicle (Fig. 1A). Treatment with spermidine also increased the plasma levels of spermidine in mice, as compared to those in vehicle-treated mice (Fig. 1B). To test the effect of spermidine in an in vivo cisplatin nephrotoxicity model, mice were orally administered various doses of spermidine before cisplatin injection, and histological and functional studies were conducted on their kidneys. Using a measurement of tubular necrosis score on PAS-stained kidney sections, the kidneys of vehicle-administered mice at 3 days after cisplatin injection showed severe tubular necrosis in the cortex and outer medulla, as represented by no nuclei, cast formation or tubular dilation, whereas cisplatin-induced tubular necrosis was diminished dose-dependently by spermidine administration (Fig. 1C, D). The mouse kidneys also revealed a timedependent increase in tubular necrosis score at 2 and 3 days after cisplatin injection, but the increase at both time-points was significantly reduced by spermidine administration (Fig. 1E). Similarly, the administration of spermidine attenuated kidney dysfunction in a dose-dependent manner, as demonstrated by the significant blunting of the plasma creatinine concentration in spermidine-administered mice compared to that in vehicle-administered mice (Fig. 1F). Two and three days after cisplatin injection, spermidine administration also inhibited an increase in the plasma creatinine concentration (Fig. 1G). In control kidneys, no significant difference was observed in the histological and functional studies between mouse kidneys treated with spermidine and those treated with vehicle (Fig. 1D–G), indicating spermidine resulted in no toxicity in mouse kidneys. Furthermore, when cisplatin-induced apoptosis was assessed in the mouse kidneys using the cleavage of caspase-3, the administration of spermidine did not alter the time-dependent increase in the expression of cleaved caspase-3 during cisplatin injury (Fig. 2). These data demonstrate that exogenous spermidine suppresses both tubular necrosis and kidney dysfunction, but not apoptosis, during cisplatin nephrotoxicity.
Exogenous spermidine diminishes DNA oxidative and nitrative stresses during cisplatin nephrotoxicity
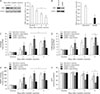
Considering that oxidative stress is involved in tubular cell death and is a critical condition for cisplatin nephrotoxicity [28], cisplatin-induced lipid peroxidation was evaluated in spermidine- or vehicle-administered mouse kidneys by measuring the lipid hydroperoxide levels. Vehicle-administered mouse kidneys showed a time-dependent increase in the lipid hydroperoxide concentrations at 1, 2, and 3 days after cisplatin, whereas spermidine administration significantly reduced the concentration of lipid hydroperoxide at 2 and 3 days after cisplatin injection, but not at 1 day (Fig. 3A). To investigate the antioxidant effect of exogenous spermidine on DNA, oxidative and nitrative stresses in DNA were next assessed by measuring the 8-OHdG and 8-nitroguanine concentrations, respectively. Cisplatin increased oxidation and nitration in DNA in vehicle-administered mouse kidneys at 1, 2, and 3 days after injection, whereas spermidine administration significantly diminished both oxidation and nitration in DNA at all time-points after cisplatin injection (Fig. 3B, C). Intriguingly, compared to the oxidative damage to lipid at 1 day after cisplatin injection (Fig. 3A), oxidative and nitrative damage to DNA at the same time after cisplatin injection was completely abolished by exogenous spermidine (Fig. 3B, C). In control kidneys, no significant alterations were shown in the oxidative/ nitrative stress in lipid and DNA between both administration of spermidine and vehicle (Fig. 3A–C). The results of oxidative and nitrative stresses suggest that the effective inhibition of DNA damage by spermidine during the early stage of cisplatin nephrotoxicity suppresses the development of functional and histological damage induced by cisplatin. Meanwhile, a subsequent reduction in the lipid damage may be implicated in the suppression of functional and histological damage.
Exogenous spermidine reduces PARP1 activation and ATP depletion during cisplatin nephrotoxicity
The excessive activation of PARP1 is considered to be a downstream effector of DNA oxidative and nitrative stresses [2930], and this further leads to tubular necrosis during cisplatin nephrotoxicity through ATP depletion induced by the poly(ADP-ribosyl)ation of various proteins, including PARP1 itself [51112]. For this reason, the PARP1 enzyme activity was measured during cisplatin nephrotoxicity in spermidine- or vehicle-administered mouse kidneys. Vehicle-administered mouse kidneys showed a marked increase in PARP1 activity at 1, 2, and 3 days after cisplatin injection, whereas spermidine administration significantly diminished the increase in PARP1 activity at all time-points (Fig. 4A). To address whether spermidine administration suppresses subsequent ATP depletion in a manner consistent with PARP1 activation, we also monitored the concentration of ATP in spermidine- or vehicle-administered mouse kidneys. Cisplatin decreased the ATP concentration in a time-dependent manner in vehicle-administered mouse kidneys at 2 and 3 days after injection, whereas spermidine administration significantly inhibited the decrease in ATP concentration during cisplatin nephrotoxicity (Fig. 4B). On the other hand, control kidneys showed no significant alteration in PARP1 activity and ATP concentration between both the administration of spermidine and vehicle (Fig. 4A, B). These data indicate that exogenous spermidine efficaciously regulates PARP1 activation and ATP depletion during cisplatin nephrotoxicity and further suggest that the effective inhibition of DNA damage by spermidine leads to the regulation of PARP1 activation.
ODC inhibition accelerates DNA damage and PARP1 activation during cisplatin nephrotoxicity
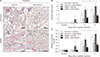
ODC catalyzes the decarboxylation of ornithine to form putrescine, producing spermidine. There is a report that cisplatin upregulates the expression of ODC mRNA in mouse kidneys [31], whereas the other report conversely reveals that the kidney expression of ODC mRNA is downregulated by cisplatin in rats [32]. Using western blot analysis, we confirmed that the expression of ODC protein in mouse kidneys was remarkably reduced at 1, 2, and 3 days after cisplatin injection (Fig. 5A). Because of that, we anticipated that spermidine depletion by ODC downregulation would accelerate DNA damage and PARP1 activation during cisplatin nephrotoxicity, finally leading to enhanced necrosis in kidney parenchymal cells. Thus, to determine whether spermidine depletion enhances oxidative stress and PARP1 activation, the genetic inhibition of ODC was induced via siRNA transfection into mice (Fig. 5B). DNA oxidation and DNA nitration in siODC-transfected mouse kidneys were worsened significantly at 1 and 2 days after cisplatin injection, compared to those in control siRNA (siControl)-transfected mouse kidneys (Fig. 5C, D). PARP1 activity in siODC-transfected mouse kidneys also increased consistently at 1 and 2 days after cisplatin injection (Fig. 5E). Intriguingly, the ODC inhibition continuously and significantly decreased ATP concentrations at 3 days as well as 1 and 2 days after cisplatin injection (Fig. 5F). These data indicate that the ODC inhibition accelerates DNA damage, PARP1 activation and ATP depletion during the early stage of cisplatin nephrotoxicity. Next, we confirmed that spermidine administration prevents the consequences induced by ODC inhibition during cisplatin nephrotoxicity. Indeed, spermidine administration significantly reduced cisplatin-induced DNA oxidation, DNA nitration, PARP1 activation and ATP depletion in siODC-transfected mouse kidneys, as much as those in siControl-transfected mouse kidneys (Fig. 5C–F). On the other hand, control kidneys showed no significant alteration in DNA oxidation, DNA nitration, PARP1 activity and ATP concentration in all of groups of siODC/siControl-transfected mouse kidneys plus/minus spermidine (Fig. 5C–F). These data indicate that not only does exogenous spermidine remove the aggravation induced by ODC inhibition during cisplatin nephrotoxicity, but it also ameliorates the consequences induced by cisplatin injury in ODC-downregulated mouse kidneys.
ODC inhibition exacerbates kidney dysfunction and tubular necrosis during cisplatin nephrotoxicity
Finally, we assessed whether ODC inhibition-induced accelerations in DNA damage, PARP1 activation and ATP depletion worsen tubular necrosis and kidney dysfunction during cisplatin nephrotoxicity. Tubular necrosis was increased more in siODC-transfected mouse kidneys than that in siControl-transfected mouse kidneys at 1, 2, and 3 days after cisplatin injection (Fig. 6A, B). The plasma creatinine concentration in siODC-transfected mice was also significantly enhanced more at 1 and 2 days after cisplatin injection compared to that in siControl-transfected mice (Fig. 6C), indicating the worsened kidney dysfunction by ODC inhibition. In sum, these data indicate that spermidine depletion induced by ODC inhibition exacerbates tubular necrosis and kidney dysfunction after cisplatin injection. Regarding spermidine administration, cisplatin-induced levels of tubular necrosis and plasma creatinine were significantly diminished by exogenous spermidine in siODC-transfected mice, as much as those in siControl-transfected mice (Fig. 6B, C). In control kidneys, no significant alteration was shown in tubular necrosis and plasma creatinine concentration in all groups of siODC/siControl-transfected mouse kidneys plus/minus spermidine (Fig. 6B, C). Consistent with data on 8-OHdG, 8-nitroguanine, PARP1 activity and ATP concentration as shown in Fig. 5, these data in Fig. 6 indicates that ODC inhibition accelerates tubular necrosis and kidney dysfunction after cisplatin injection, whereas exogenous spermidine abrogates the consequences of both ODC inhibition and cisplatin nephrotoxicity.
Discussion
Cisplatin nephrotoxicity is associated with tubular cell death through a combination of various conditions including DNA damage, plasma membrane disruption, mitochondrial dysfunction, and oxidative stress [4]. In spite of having been the focus of active investigation for many decades, there exists a gap in our understanding of the exact pathogenesis of cisplatin nephrotoxicity. Most studies have emphasized apoptosis and inflammation, which are considered to be the major contributors of cisplatin injury [2]. Meanwhile, pathological reports of non-apoptotic cell death in patients with toxic kidney failure are sparse [4]. Although recent studies have demonstrated that necrotic signal transduction pathways are required for cisplatin nephrotoxicity [1233], the extent to which necrosis contributes to the pathophysiology of cisplatin-induced acute kidney injury is still questioned. Our present data reveal a novel inhibition of tubular necrosis by spermidine to prevent cisplatin nephrotoxicity, suggesting that necrosis is a major determinant of cisplatin nephrotoxicity.
Oxidative and nitrative stresses react with lipids, nucleic acids, proteins and carbohydrates to contribute to the development of necrosis. In particular, DNA is believed to be the primary biological target of cisplatin, which binds covalently to purines to form intrastrand and interstrand crosslinks [34] and subsequently increases oxidative and nitrative stresses. Antioxidant and antinitrative actions have been investigated as potential protection against cisplatin nephrotoxicity. For example, manganese superoxide dismutase overexpression and treatment with a superoxide dismutase mimetic molecule attenuate cisplatin-induced kidney tubular injury by suppressing the generation of oxidative stress [3536]. The inhibition of nitric oxide synthase and peroxynitrite also reduces cisplatin-induced kidney tubular injury by preventing lipid peroxidation and antioxidant enzyme downregulation [373839]. The present study supports these reports by demonstrating that spermidine administration potently inhibits oxidative and nitrative stresses during cisplatin nephrotoxicity and subsequently protects against cisplatin-induced tubular necrosis. Thus, oxidative/nitrative stress-induced necrotic process would be of cardinal importance in understanding the mechanism of cisplatin nephrotoxicity.
The renoprotective effect of spermidine against cisplatin nephrotoxicity is manifested through the effective inhibition of DNA damage in an early stage after cisplatin injection. The present study shows that oxidative and nitrative DNA damage in kidneys was prevented at 1 day after cisplatin injection, whereas lipid peroxidation in kidneys was not altered at the same time. Lipid peroxidation products including malondialdehyde and 4-hydroxynonenal typically induce DNA oxidation and DNA nitration [40], but reactive oxygen/nitrogen species generated by direct interaction with cisplatin or irradiation in DNA induce lipid peroxidation, leading to cell death by necrosis and apoptosis [4142]. This theory supports the suggestion in the present study that a significant reduction of oxidative/nitrative DNA damage obtained with spermidine at 1 day after cisplatin injection affects the subsequent reduction of lipid peroxidation at 2 and 3 days after cisplatin injection.
Spermidine suppresses necrotic cell death in eukaryotic cells. In yeast and human immune cells, ageing-associated necrosis is attenuated by spermidine treatment, but apoptosis is not altered by spermidine [16]. In contrast with apoptotic cell death that is characterized by DNA fragmentation and cell shrinkage, necrotic cell death morphologically indicates ATP depletion and plasma membrane disruption. Finally, necrotic cells release intracellular proteins such as high-mobility group box 1 and lead to inflammation in neighboring cells [1143]. PARP1, an important factor in various pathogeneses, is considered a downstream effector of oxidative and nitrative stresses [4445]. Although PARP1 acts as a key enzyme in detecting and repairing DNA, an excessive activation of PARP1 induced by oxidative/nitrative DNA damage leads to pathological necrotic cell death through ATP depletion [4647]. In previous studies, we have already demonstrated that genetic or pharmacological inhibition of PARP1 protects against cisplatin nephrotoxicity [5101112]. Taken together, the present data demonstrate that the renoprotective effect of spermidine against cisplatin-induced tubular necrosis is implicated in the inhibition of PARP1 activation through the reduction of DNA damage.
In particular, the present study has shown that ODC inhibition induced by siRNA transfection in vivo makes the kidneys more susceptible to cisplatin nephrotoxicity. As previously reported, ODC deficient yeast has been unable to synthesize polyamines as confirmed by measurement of intracellular spermidine [16]. A specific irreversible inhibitor of ODC, difluoromethylornithine, also has depleted spermidine and further induced cell death in various cell types [48]. Consistent with previous reports, ODC inhibition further enhances DNA damage, PARP1 activation, ATP depletion and subsequent tubular necrosis during cisplatin nephrotoxicity compared to those in siControl-transfected mouse kidneys.
In summary, the present study has shown that spermidine regulates DNA damage and PARP1 activation in cisplatin nephrotoxicity and results in renoprotection against tubular necrosis and kidney dysfunction. Future studies are evaluate the potential kidney health-promoting effects of spermidineenriched diets and will further reveal whether spermidine or its agent has beneficial effects on various kidney diseases.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




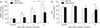
 XML Download
XML Download