Dear Editor,
Multiple sclerosis (MS) frequently affects women of child-bearing age.1 When selecting appropriate disease-modifying therapies (DMTs) for MS patients, practical issues about pregnancy should be considered for individual patients since no DMTs have been proven to be safe during pregnancy.1
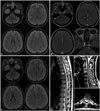
A 28-year-old woman was admitted due to decreased visual acuity in September 2015. A neurological examination revealed decreased visual acuity in both eyes, a relative afferent pupillary defect in the right eye, bilateral optic disc swelling (which was more severe on the right), and paresthesia in the lower extremities. Initial brain and spine MRI images showed multifocal lesions with or without gadolinium enhancement in the cerebral hemisphere, the brainstem, both optic nerves, and the spinal cord, which were compatible with the McDonald and the recent magnetic resonance imaging in multiple sclerosis (MAGNIMS) criteria (Fig. 1).23
After the initial high-dose steroid therapy, teriflunomide (14 mg) was started after explaining the need for contraception. She already had two children and denied having any plans for future childbirth. Follow-up MRI in December 2015 revealed that the previous lesions had almost resolved (Fig. 1). She had no clinical worsening of MS, and the DMT with teriflunomide was continued. In February 2016 she visited an outpatient clinic due to unplanned pregnancy. The obstetric ultrasound examination indicated that fetus was at a gestational age of 6 weeks. Teriflunomide was stopped immediately and the patient was recommended to receive an accelerated elimination procedure. However, she declined to undergo accelerated elimination and wanted to continue the pregnancy. During the pregnancy, the patient regularly visited the outpatient clinic without any DMT, and no clinical worsening of MS was indicated. Also, regular obstetric examinations did not reveal any abnormality of the fetus or mother. She delivered a healthy baby via a normal vaginal delivery in October 2016. A neurological examination and follow-up MRI performed in November 2016 found no evidence of relapse. In December 2016, teriflunomide was restarted since the patient complained of sensory disturbance on the extremities. During regular visits to our clinic the patient showed no signs of clinical worsening. The baby showed normal developmental milestones for physical, intellectual, and social-emotional growth over the past 18 months.
Teriflunomide was initially approved in 2012, was previously categorized as X by the US FDA, and is now described as being contraindicated in pregnant women, and it has been found to be a teratogenic drug in experimental studies with rodents and rabbits. Several previous studies even suggested that a negative pregnancy test and adequate contraception are both required in women of child-bearing potential who will be taking teriflunomide.1 A few structural abnormalities (i.e., ureteropyeloectasia and congenital hydrocephalus) have been found in clinical studies and the postmarketing data of teriflunomide,4 but the overall pregnancy outcomes including the rate of spontaneous abortions and malformations were similar to those observed in the general population.4
There have been 26 cases of reported pregnancies during teriflunomide treatment, and accelerated elimination procedures were performed in 22 of them.56 The use of activated charcoal or cholestyramine for accelerated elimination is generally relatively safe even in pregnant women, with only minor side effects such as mild nausea.7 In the present case we could not perform an accelerated elimination procedure. However, the baby and mother were healthy in 4 of the 26 previous cases that did not have accelerated elimination procedures.56
The present study has the limitation of involving a single case, and this may be a unique report of a safe pregnancy in an East Asian patient taking teriflunomide. This case adds to the clinical experience related to safety issues of teriflunomide in pregnancy. However, while taking teriflunomide, all women of reproductive age should be counseled about the possibility of pregnancy. Also, accelerated elimination is recommended as soon as an unplanned pregnancy is discovered since teriflunomide is still contraindicated in pregnancy
Figures and Tables
Fig. 1
MRI scans of the patient. Initial brain MRIs show multiple HSILs in the pons, middle cerebellar peduncle, subcortical and periventricular white matter on the FLAIR imaging (A) and gadolinium-enhancement imaging (B). B: The optic nerves are enhanced bilaterally. C: Three months after administration of teriflunomide, follow-up FLAIR images show resolution of previous HSILs. D: The spine MRIs show eccentric HSILs in the thoracic (arrows) and the cervical spinal cord (open arrow). HSILs: high signal intensity lesions.
References
1. Cree BA. Update on reproductive safety of current and emerging disease-modifying therapies for multiple sclerosis. Mult Scler. 2013; 19:835–843.

2. Filippi M, Rocca MA, Ciccarelli O, De Stefano N, Evangelou N, Kappos L, et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol. 2016; 15:292–303.

3. Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011; 69:292–302.

4. Vukusic S, Coyle PK, Jurgensen S, Truffinet P, Benamor M, Poole E, et al. Pregnancy outcomes in patients with MS treated with teriflunomide: clinical study and postmarketing data. Neurology. 2018; 90:15 supplement. P4.361.
5. Confavreux C, O'Connor P, Comi G, Freedman MS, Miller AE, Olsson TP, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014; 13:247–256.

6. Kieseier BC, Benamor M. Pregnancy outcomes following maternal and paternal exposure to teriflunomide during treatment for relapsing-remitting multiple sclerosis. Neurol Ther. 2014; 3:133–138.

7. Position statement and practice guidelines on the use of multi-dose activated charcoal in the treatment of acute poisoning. J Toxicol Clin Toxicol. 1999; 37:731–751.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download