Dear Editor,
GNE myopathy is an autosomal recessive myopathy that was previously known as distal myopathy with rimmed vacuoles (OMIM 605820) or hereditary inclusion-body myopathy (OMIM 600737). GNE myopathy is clinically characterized by early involvement of the tibialis anterior muscle leading to early foot drop, with relatively spared quadriceps muscles.1 However, diagnosing GNE myopathy can be challenging due to its phenotypic variability.
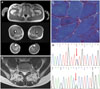
A 23-year-old man presented with back pain. He had been serving in the military service for 6 months and his back pain started after strenuous activities involving the back muscles. The initial physical examination revealed back extension weakness and abdominal muscle weakness (MRC grade 3). He was unable to perform a single sit-up. The patient had mild ankle dorsiflexion weakness (MRC grade 4+) that he did not recognize and which did not affect his self-ambulation ability. Moreover, the patient showed no muscular atrophy in the legs. He had a younger brother who was healthy, and his parents also had no past history of any disease. The initial laboratory data were unremarkable except for elevated creatinine kinase (1,146 IU/L). The findings of a nerve conduction study were normal, while electromyography showed evidence of active myopathy, with active denervation potentials observed in the right tibialis anterior, medial gastrocnemius, and thoracic and lumbar paraspinal muscles. Muscle T1-weighted MRI revealed mild fatty infiltrative and atrophic changes in the bilateral hamstring and soleus muscles (Fig. 1A). There was marked fatty infiltration in the lumbar paraspinal muscles but without significant nerve compression that could cause back pain (Fig. 1B). The cervical and thoracic paraspinal axial muscles were intact. A pathologic investigation of the left tibialis anterior muscle revealed mild fiber size variations without significant inflammatory infiltrates and rimmed vacuoles that were suggestive of GNE myopathy (Fig. 1C). A genetic study revealed a pair of compound heterozygous missense mutations in GNE (p.V572L and p.A591T; Fig. 1D) that have previously been reported (NP_005467.1) to be among the most-common GNE mutations found in Korean and Japanese subjects.
The decreased sialic acid synthesis due to a biallelic mutation in UDP N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase that leads to decreased GNE activity is known to cause GNE myopathy.2 However, the exact mechanism of GNE alteration leading to muscle atrophy remains to be elucidated.34 The disease is characterized by progressive weakness that predominates in the distal muscles. The early involvement of the tibialis anterior muscle with relative sparing of the quadriceps muscles are well-known features of GNE myopathy. However, recent studies reported the early involvement in the proximal lower limbs, mimicking limb-girdle muscular dystrophy.56 In addition, clinical heterogeneities have been observed for the same mutations.7 Heterozygous mutations of p.V572L and p.A591T (the same mutations as in our patient) were reported previously, in which the phenotype was a classic pattern of distal leg weakness and quite different from the present case.7 These findings indicate that the genotype–phenotype correlation in GNE myopathy is not distinct.
There is a recent report of a young male patient who presented with asymmetric hand weakness.8 The patient was an endoscopist with strenuous and repetitive use of his left hand. The report described that this patient experienced pain and weakness in the left hand, which was confirmed as GNE myopathy with asymmetric upper limb involvement. Similarly, our patient developed back pain and weakness after repetitive strenuous activities. Although caution is prudent when attributing axial muscle weakness solely to training, the clinical history of axial muscle overuse of the patient may be an extrapolating cause of the atypical clinical presentation. Therefore, more studies are warranted to elucidate the relationship between muscle overuse and atrophy.
Figures and Tables
Fig. 1
MRI scans, histopathology findings, and GNE mutations of the patient. A: Lower extremity T1-weighted MRI showed asymmetric fatty infiltration of the posterior compartments of the thigh and calf muscles. B: The most-striking change was severe fatty infiltration and atrophy of the lumbar paraspinal muscles in T2-weighted MRI imaging (arrows). C: A few rimmed vacuoles and minor variations in fiber sizes without significant inflammatory infiltrates were evident in modified Gomori trichrome stain, ×200. D: Sequencing chromatograms of c.1714G>C (p.V572L) and c.1771G>A (p.A591T) in GNE (arrows indicate the mutation sites).
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (No. 2017R1C1B5076264).
References
1. Argov Z, Yarom R. “Rimmed vacuole myopathy” sparing the quadriceps. A unique disorder in Iranian Jews. J Neurol Sci. 1984; 64:33–43.
2. Gagiannis D, Orthmann A, Danssmann I, Schwarzkopf M, Weidemann W, Horstkorte R. Reduced sialylation status in UDP-N-acetylglucosamine-2-epimerase/N-acetylmannosamine kinase (GNE)-deficient mice. Glycoconj J. 2007; 24:125–130.

3. Nishino I, Carrillo-Carrasco N, Argov Z. GNE myopathy: current update and future therapy. J Neurol Neurosurg Psychiatry. 2015; 86:385–392.

4. Penner J, Mantey LR, Elgavish S, Ghaderi D, Cirak S, Berger M, et al. Influence of UDP-GlcNAc 2-epimerase/ManNAc kinase mutant proteins on hereditary inclusion body myopathy. Biochemistry. 2006; 45:2968–2977.

5. Park YE, Kim HS, Choi ES, Shin JH, Kim SY, Son EH, et al. Limb-girdle phenotype is frequent in patients with myopathy associated with GNE mutations. J Neurol Sci. 2012; 321:77–81.

6. Argov Z, Eisenberg I, Grabov-Nardini G, Sadeh M, Wirguin I, Soffer D, et al. Hereditary inclusion body myopathy: the Middle Eastern genetic cluster. Neurology. 2003; 60:1519–1523.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download