Abstract
Background
Bendamustine is a chemotherapeutic agent that has shown broad activity in patients with lymphoid malignancies. It contains both alkylating and nucleoside analog moieties, and thus, is not commonly used for stem cell mobilization due to concerns that it may adversely affect stem cell collection. Here we describe the lymphoma subset of a prospective, non-randomized phase II study of bendamustine, etoposide, and dexamethasone (BED) as a mobilization agent for lymphoid malignancies.
Methods
This subset analysis includes diffuse large B-cell lymphoma (N=3), follicular lymphoma (N=1), primary mediastinal B-cell lymphoma (N=1), and NK/T-cell lymphoma (N=1). Patients received bendamustine (120 mg/m2 IV d 1, 2), etoposide (200 mg/m2 IV d 1–3), and dexamethasone (40 mg PO d 1–4) followed by filgrastim (10 mcg/kg/d sc. through collection).
Results
We successfully collected stem cells from all patients, with a median of 7.9×106/kg of body weight (range, 4.4 to 17.3×106/kg) over a median of 1.5 days (range, 1 to 3) of apheresis. All patients who received transplants were engrafted using kinetics that were comparable to those of other mobilization regimens. Three non-hematologic significant adverse events were observed in one patient, and included bacterial sepsis (grade 3), tumor lysis syndrome (grade 3), and disease progression (grade 5).
Autologous stem cell transplantation (ASCT) is a standard of care for patients with refractory non-Hodgkin lymphoma (NHL) [1]. Bendamustine (Treanda, Teva Pharmaceuticals, Petach Tikva, Israel) is a nitrogen mustard chemotherapeutic agent with a unique mechanism of action. It has both alkylating and anti-metabolite properties, which may allow it to overcome cross-resistance to many other chemotherapeutic agents [23]. Indeed, bendamustine-based regimens can salvage relapsed indolent, diffuse large B-cell, chronic lymphocytic, and T-cell lymphomas [4567]. When bendamustine is used as a single chemomobilization agent for peripheral blood stem cell (PBSC) mobilization, it results in relatively poor and unpredictable mobilization, even when combined with G-CSF or plerixafor [8]. In order to overcome this limitation, we chose to combine bendamustine with etoposide, which is an effective mobilization agent for patients with refractory lymphoma, and the addition of etoposide to bendamustine can overcome the poor mobilization outcomes of bendamustine alone [910]. We previously showed that a bendamustine-based regimen is effective for mobilization in multiple myeloma [11]. Bendamustine has been used for cytoreduction in lymphoma, followed by mobilization with a different agent [1213]. However, no prospective studies have investigated the safety and efficacy of full-dose bendamustine as a stem cell mobilization agent in lymphoma before ASCT. Here, we report the outcomes of patients with lymphoma who were enrolled in the phase II BED mobilization trial.
This single-center, open-label prospective trial was open to patients with lymphoid malignancies who were planning to undergo ASCT. This trial was approved by the Fred Hutchinson Cancer Research Center/University of Washington Cancer Consortium Institutional Review Board, and written informed consent was obtained from all patients. Eligibility criteria included an ECOG status <2, absolute neutrophils counts >1.5×109/L, platelet counts >100×108/L, creatinine clearance greater than 50 mL/min (Cockcroft-Gault formula), bilirubin <1.5 times the upper limit of normal (ULN), and AST and ALT <2.5 times ULN. Patients were excluded if they had prior resistance to bendamustine, >4 prior different myelotoxic chemotherapy regimens (e.g. ICE, DHAP, MTX/HiDAC, hyperCVAD), symptomatic cardiopulmonary disease, fludarabine therapy in the preceding 24 months, a prior failed stem cell mobilization attempt, a prior autologous or allogeneic stem cell transplant, known HIV, hepatitis B or C, >three cycles of multi-agent myelotoxic salvage chemotherapy within four months of enrollment, prior pelvic/spinal irradiation, or systemic chemotherapy within three weeks of initiating BED.
Patients were administered one cycle of BED [bendamustine (120 mg/m2 IV d 1, 2), etoposide (200 mg/m2 IV d 1–3), dexamethasone (40 mg PO d 1–4), delivered in an outpatient setting, followed by filgrastim (initially 10 mcg/kg/d sc, starting on day 5 through end of collection)]. Apheresis was initiated when peripheral blood CD34+ cell counts were >5/µL. The primary endpoint was successful mobilization in over 80% of patients, which was defined as the collection of >2.0×106 CD34+ cells/kg. Adverse events (AEs) were graded using the common terminology criteria of adverse events (CTCAE) v4.0. Secondary endpoints included the number of apheresis cycles that were required to collect a minimum of >2×106 CD34+ cells/kg (and ideally >5×106 CD34+ cells/kg) and disease response rate to one cycle of BED. When patients' absolute neutrophil counts dropped below 500/µL, prophylactic antibiotic therapy (fluoroquinolone) was initiated at the discretion of the treating physician. Standard Center for International Blood and Marrow Transplant Research (CIBMTR) criteria were used for assessing blood count recovery.
The response in patients with measurable disease was a secondary endpoint and was assessed after a single cycle of BED. Disease definitions, evaluation criteria, endpoint definitions, and response criteria were defined by the standard NCI criteria for lymphoid malignancies [14].
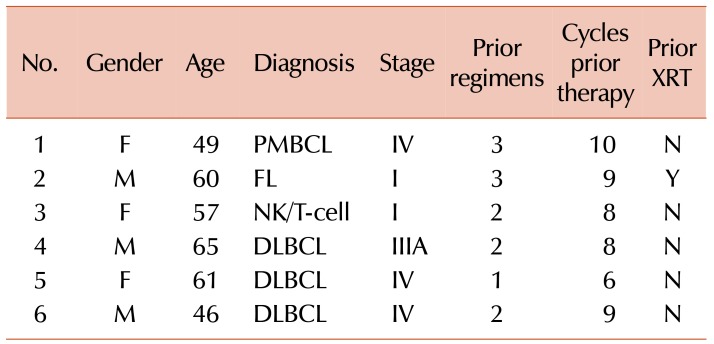
From July 2011 to September 2013, we enrolled six patients with lymphoma, three of whom had diffuse large B-cell lymphoma, one with primary mediastinal B-cell lymphoma, one with follicular lymphoma, and one with NK/T-cell lymphoma. The patient characteristics are described in Table 1. Patients were a median age of 58.5 years (range, 46–65). The patients had received a median of two lines of prior cytotoxic therapy (range, 1–3) with a median number of 8.5 cycles (range, 6–9), and one patient had received prior radiotherapy. Three of the patients had chemorefractory lymphoma with residual disease at enrollment, after their most recent cycle of prior therapy (Table 2).
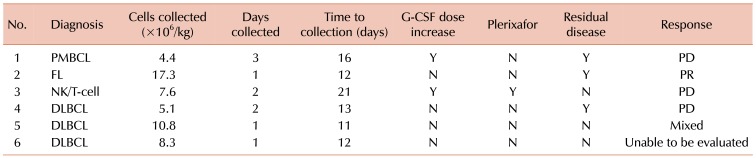
All patients were successfully mobilized, and no chemotherapy dose reductions were required. The median number of CD34+ cells collected was 7.9×106/kg (range, 4.35 to 17.3×106). Stem cells were collected at a median of 13 days (range, 11–21) after the start of mobilization. The median number of apheresis days was 1.5 (range, 1–3). The patient with NK/T cell lymphoma received an increased dose of G-CSF, to 16 mcg/kg/bid, due to prolonged neutropenia and a concurrent infection. This patient ultimately received plerixafor on days 20 and 21 after BED and successfully mobilized adequate stem cells (Table 2). Furthermore, this patient had previously received four cycles of vincristine/doxorubicin/cyclophosphamide, followed by four courses of high dose methotrexate/dexamethasone/ifosfamide/etoposide/L-asparginase. The patient with primary mediastinal B-cell lymphoma also received a G-CSF dose increase to reduce the risk of infection in the setting of neutropenia. This patient had previously received six cycles of R-CHOP, three cycles of R-ICE, and one cycle of ESHAP before proceeding to BED mobilization.
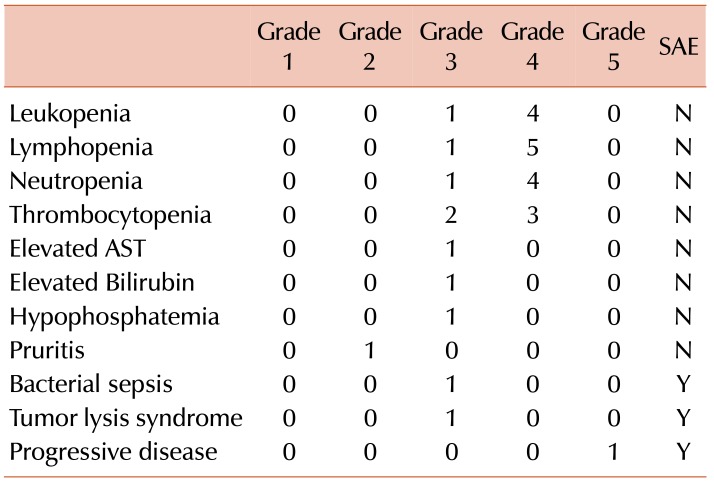
Expected grade 3 or 4 leukopenia, neutropenia, lymphopenia, and thrombocytopenia were seen in most patients. The patient with NK-T cell lymphoma experienced all of the observed significant adverse events (SAEs) in the study and subsequently died due to disease progression prior to ASCT. The SAEs are listed in Table 3 and included neutropenic fever (grade 3), tumor lysis syndrome (grade 3), and disease progression (grade 5). Post-transplant, the patients achieved an unsupported neutrophil count >500/µL at a median of 15 days (range, 13–21), and a platelet count >200,000/µL at a median of 11 days (range, 8–12).
Among the patients with measurable disease prior to chemomobilization, all patients who responded had chemosensitive disease. One patient with DLBCL showed a mixed response, and this patient had a CR to R-CHOP but relapsed prior to mobilization. The patient with high grade follicular lymphoma had a partial response (PR) to BED. This patient had a CR to R-CHOP, a CR to R-EPOCH, but developed progressive disease (PD) on maintenance rituximab prior to chemomobilization. The other patients did not demonstrate reduction in disease burden. One patient with DLBCL had no measurable disease prior to chemomobilization and thus could not have response assessed. Prior to mobilization, this patient had received a complete response (CR) to R-CHOP, relapsed, and then achieved a CR to R-ICE. He remained in remission after chemomobilization.
Decreasing lymphoma burden prior to transplant has been shown to improve transplant outcomes [15]. Currently, salvage chemotherapy is commonly used for mobilization, and the most common regimen is ifosfamide, etoposide, and carboplatin (ICE) [16]. While etoposide alone is an effective mobilization agent, many patients have been exposed to this drug during prior cytotoxic chemotherapy. For diseases that are refractory to these chemotherapies, there is an unmet need for mobilization agents that do not have cross resistance.
Bendamustine is an attractive agent for mobilization because it may have less cross-resistance to other agents. However, there are concerns that it may adversely affect stem cell collection or engraftment. Here, we show that mobilization with a single cycle of BED in non-Hodgkin lymphoma is sufficiently safe and effective, even in patients who are heavily pre-treated. All patients met the primary endpoint of adequate CD34+ cell mobilization, with a median of 7.9×106 cells/kg. Two of the six patients required increased doses of G-CSF, and one required plerixafor. Adequate stem cell collection required a median of 1.5 days of apheresis. This compares favorably to ICE±rituximab with routine G-CSF, which yields cell numbers of 5.07×106 cells/kg over a median of two apheresis days. Additionally, the toxicity that has been associated with BED is similar to that of this regimen [17]. The addition of bendamustine to etoposide also has improved effects over single agent bendamustine, which only mobilizes poorly, even when G-CSF and plerixafor are administered [8].
All of the patients who received transplants engrafted within 21 days. As part of the larger phase II trial, we showed that BED was effective for mobilization in multiple myeloma [11]. This study extends those results to demonstrate that that BED can also be successfully used for mobilization in non-Hodgkin lymphoma.
We also evaluated the efficacy of BED as a secondary endpoint. After a single cycle of this regimen, we only observed anti-tumor activity in patients who were previously responsive to the prior line of therapy. Of the patients who responded to the prior line of therapy, one had a partial response, one had a mixed response, and one had progressive disease. Responses to BED were only seen in DLBCL and high grade follicular lymphoma. The major caveats to our observations include the small number of patients who were included in our series and the limited comparative data that originated from single cycle regimens in patients with lymphoma. Nevertheless, our data do not support the use of this approach to control the most chemorefractory lymphoma.
The majority of AEs were mild, most of which were the expected cytopenias of chemotherapy. These are similar to the AEs that were observed in a larger subset of the phase II trial, which included patients with multiple myeloma [11]. A single patient experienced all of the SAEs and developed sepsis and died during the trial. This patient developed progressive disease during the trial, and these adverse events were considered to be secondary to the disease, rather than to the chemotherapy regimen.
In conclusion, peripheral blood stem cell mobilization with BED is comparable to other mobilization regimens in terms of safety, effectiveness, and time to engraftment. Although this study is limited by its small sample size, the use of bendamustine for mobilization warrants further studies in the appropriate patient populations.
ACKNOWLEDGMENTS
Teva Pharmaceutical Industries, NCI K08 CA151682 (D.J.G.), NCI P01CA44991, NCI R01CA076287, NCI R01 CA138720, 1K24CA184039, NCI T32CA009515. Fred Hutchinson Cancer Research Center/University of Washington Cancer Consortium Cancer Center Support Grant P30 CA015704 and philanthropic gifts from Frank and Betty Vandermeer. AKG is a Scholar in Clinical Research for the Leukemia and Lymphoma Society.
Notes
This study was supported by Teva Pharmaceuticals. The funding was used to support the salaries of the research staff. Bendamustine was provided to patients by Teva Pharmaceuticals at no cost. Teva Pharmaceuticals played no role in the study design, the collection and analysis of the data, the decision to publish this work, or the writing of this manuscript. Drs. Green, Gopal, and Budde have received research support from Teva Pharmaceuticals.
References
1. Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995; 333:1540–1545. PMID: 7477169.

2. Leoni LM, Hartley JA. Mechanism of action: the unique pattern of bendamustine-induced cytotoxicity. Semin Hematol. 2011; 48:S12–S23. PMID: 21530768.

3. Leoni LM, Bailey B, Reifert J, et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res. 2008; 14:309–317. PMID: 18172283.

4. Robinson KS, Williams ME, van der Jagt RH, et al. Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin's lymphoma. J Clin Oncol. 2008; 26:4473–4479. PMID: 18626004.

5. Ohmachi K, Niitsu N, Uchida T, et al. Multicenter phase II study of bendamustine plus rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2013; 31:2103–2109. PMID: 23650408.

6. Fischer K, Cramer P, Busch R, et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2011; 29:3559–3566. PMID: 21844497.

7. Damaj G, Gressin R, Bouabdallah K, et al. Results from a prospective, open-label, phase II trial of bendamustine in refractory or relapsed T-cell lymphomas: the BENTLY trial. J Clin Oncol. 2013; 31:104–110. PMID: 23109692.

8. Gac AC, Azar N, Daguindau E, et al. Does bendamustine impact the mobilization of peripheral blood stem cells? A multicenter retrospective study of 23 cases. Leuk Lymphoma. 2016; 57:1149–1153. PMID: 26879408.

9. Reiser M, Josting A, Draube A, et al. Successful peripheral blood stem cell mobilization with etoposide (VP-16) in patients with relapsed or resistant lymphoma who failed cyclophosphamide mobilization. Bone Marrow Transplant. 1999; 23:1223–1228. PMID: 10414907.

10. Green DJ, Bensinger WI, Holmberg L, et al. Bendamustine (Treanda®), etoposide and dexamethasone (BED) followed by GCSF effectively mobilizes autologous peripheral blood hematopoietic stem cells. Blood (ASH Annual Meeting Abstracts). 2012; 120(Suppl):abst 4126.

11. Green DJ, Bensinger WI, Holmberg LA, et al. Bendamustine, etoposide and dexamethasone to mobilize peripheral blood hematopoietic stem cells for autologous transplantation in patients with multiple myeloma. Bone Marrow Transplant. 2016; 51:1330–1336. PMID: 27214069.

12. Chen RW, Li H, Bernstein SH, et al. RB but not R-HCVAD is a feasible induction regimen prior to auto-HCT in frontline MCL: results of SWOG Study S1106. Br J Haematol. 2017; 176:759–769. PMID: 27992063.

13. Armand P, Redd R, Bsat J, et al. A phase 2 study of rituximab-bendamustine and rituximab-cytarabine for transplant-eligible patients with mantle cell lymphoma. Br J Haematol. 2016; 173:89–95. PMID: 26729345.

14. Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007; 25:579–586. PMID: 17242396.
15. Cowan AJ, Stevenson PA, Cassaday RD, et al. Pretransplantation minimal residual disease predicts survival in patients with mantle cell lymphoma undergoing autologous stem cell transplantation in complete remission. Biol Blood Marrow Transplant. 2016; 22:380–385. PMID: 26348890.

16. Kewalramani T, Zelenetz AD, Nimer SD, et al. Rituximab and ICE as second-line therapy before autologous stem cell transplantation for relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2004; 103:3684–3688. PMID: 14739217.

17. Fox CP, McMillan AK, Bishton MJ, Haynes AP, Russell NH. IVE (ifosfamide, epirubicin and etoposide) is a more effective stem cell mobilisation regimen than ICE (ifosphamide, carboplatin and etoposide) in the context of salvage therapy for lymphoma. Br J Haematol. 2008; 141:244–248. PMID: 18353164.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download