Abstract
Background
This study aimed to evaluate the prognostic value of smudge cell percentage as a surrogate marker for zeta-chain-associated protein kinase 70 (ZAP-70) expression in chronic lymphocytic leukemia (CLL) patients.
Methods
Sixty three newly diagnosed CLL patients were investigated at the Hematology Department of the Medical Research Institute of Alexandria University with complete blood count, lactate dehydrogenase, β2 microglobulin levels, ZAP-70 expression, and estimation of the percentage of smudge cells.
Results
The percentage of smudge cells ranged from 2 to 58% with a mean of 24.03±13.98%. Higher percentages of smudge cells (>30%) were statistically significantly associated with markers of better prognosis (negative ZAP-70, early-stage disease according to the Binet and Rai staging systems, as well as low and intermediate risk CLL prognostic index). The percentage of smudge cells showed significantly negative correlation with the ZAP-70 expression and higher area under the curve for prediction of ZAP-70 positivity with better survival for 36 months in patients with >30% smudge cells.
Chronic lymphocytic leukemia (CLL) is a clonal progressive accumulation of mature small B-lymphocytes with a narrow rim of cytoplasm [1]. The peripheral blood of CLL patients may include other lymphocytes with atypical morphology such as small lymphocytes with cleaved nuclei, large immature lymphocytes with abundant cytoplasm (prolymphocytes), large granular lymphocytes, and T-lymphocytes [23]. Characteristic disintegrated or crushed white cells are present in the blood film of CLL patients called smudge cells (SCs) or basket cells. They were first described in 1896 by Gumprecht as white blood cells with broken-down nuclei (Gumprecht's nuclear shadows) [4].
These cells smudge when blood films are made, probably because they are more fragile than normal cells and prone to traumatic disruption during blood film preparation [5]. However, the exact mechanism by which SCs appear in the peripheral blood film is not completely understood.
SCs can occasionally be observed in normal peripheral blood films (PBFs) [6]. They can also be seen with a variable number in abnormal PBFs such as those of patients with acute and chronic leukemia but usually in greater numbers in CLL [7].
Evaluation of prognostic markers in CLL patients is an important step in selecting the appropriate management strategy for such patients. One of the standard prognostic markers is the zeta-chain-associated protein kinase 70 (ZAP-70) expression. In this study, newly diagnosed CLL patients were evaluated for the presence of any significant relationship between the percentage of SCs in PBFs and the established prognostic markers in CLL patients, looking for the possibility of using SC percentage as a surrogate marker for ZAP-70 expression.
This prospective study included 63 newly diagnosed CLL patients in the Hematology Department of the Medical Research Institute, Alexandria University for 3 years (from Jan. 1st, 2014 to Dec. 31st, 2016).
Patients were physically examined and investigated with complete blood count, lactate dehydrogenase, β2 microglobulin levels; imaging for staging (Rai and Binet staging systems) [89], CLL prognostic index [10], and flow cytometry to confirm CLL diagnosis and to determine the ZAP-70 expression [Becton Dickinson, FACSCalibur flow cytometer equipped with BD CellQuest Pro software (BD Biosciences, San Jose, CA, USA)]. All the included 63 patients had CLL immunophenotypic scores of 4 or 5.
For the analysis of ZAP-70 expression, blood samples were incubated with anti-CD5-FITC and anti-CD19-PerCP-Cy5.5 (Immunotech, Beckman Coulter) for 15 min; red cells were lysed using IO Test 3 lysing solution (Beckman Coulter, Fullerton, USA). ZAP-70 is an intracytoplasmic antigen, so its detection requires an extra step of permeabilization. After centrifugation at 200 g, fixation and permeabilization of cells were carried out using 4% paraformaldehyde and 2% Tween 20, Intraprep kits reagent (Immunotech, Beckman Coulter); anti-ZAP-70-PE (clone SBZAP) (Beckman Coulter, Fullerton, California, USA) was then added and cells were incubated for 15 min, cells were washed with phosphate-buffered saline, and analyzed by flow cytometry. After gating on B cells (CD5+/CD19+), percentages of ZAP-70-positive CLL cells were determined with negative threshold cutoff values set using ZAP-70-stained normal B cells, as well as isotype control-stained CLL cells. The cut-off point for ZAP-70 positivity in CLL cells was more than 20% [11].
According to Matutes et al. (1994) [12] and Moreau et al. (1997) [13], each one of the following: expression of CD23, CD5, the absence of FMC7 with weak CD79b and SmIg scored a point. A total score of ≥4 had an accuracy of 97% for the diagnosis of CLL.
Peripheral blood films were manually smeared directly after venipuncture before the addition of EDTA, stained with Leishman stain and the SC percentage was estimated by counting 100 lymphocytes and/or SCs; the SC number was then considered as a proportion of SCs per 100 intact lymphocytes. SCs were defined as broken cells with no intact cytoplasms and disrupted nuclear membranes.
To facilitate the objective of this study, the SC percentage was categorized as ≤30% versus >30%. The application of 30% threshold showed the best results in stratifying outcome in an independent and previously reported patient series that observed a significant separation of survival curves [14].
Data were processed SPSS for Windows version 24 (IBM Corp., Armonk, NY, USA). Since the data were normally distributed (parametric), the Pearson correlation test was applied at a 5% significance level. The qualitative variables were analyzed using the chi-square test.
Receiver operating characteristic (ROC) curve analysis was then used to identify the predictive value for SC percentage in the estimation of ZAP-70 positivity among CLL patients. Finally, the Kaplan Meier overall survival analysis was computed in relation to SC percentage.
Ethically, this study was approved by the ethical committee of the Medical Research Institute of Alexandria University and written informed consent was obtained from each participant prior to inclusion.
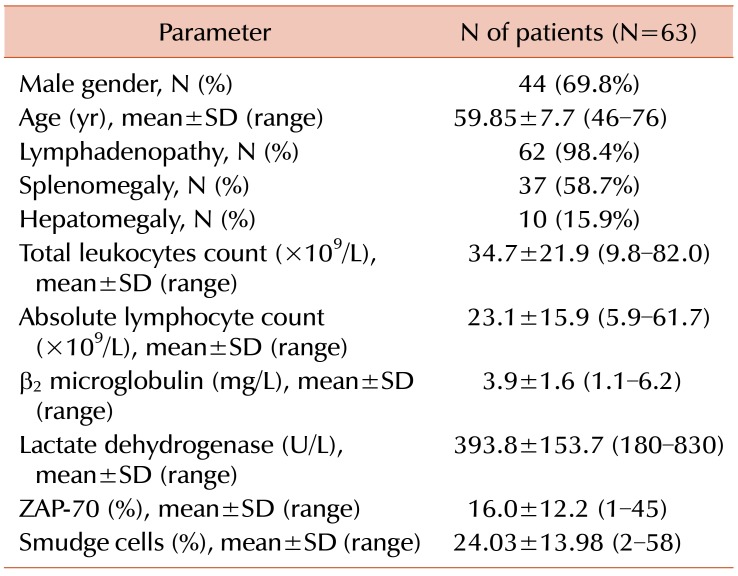
The included 63 CLL patients showed a higher frequency of male over female patients (69.8% vs. 30.2%). The male to female ratio was 2.3:1. Their ages ranged from 46 years to 76 years with a mean of 59.85±7.7 years (Table 1).
Clinical examination of the patients revealed a higher proportion of lymphadenopathy (98.4%) followed by splenomegaly (58.7%) and hepatomegaly (15.9%) (Table 1).
Hematological parameters showed higher mean total leukocytic count and higher mean absolute lymphocytic count and the percentage of SCs ranged from 2 to 58% in the studied patients with a mean of 24.03±13.98% (Table 1).
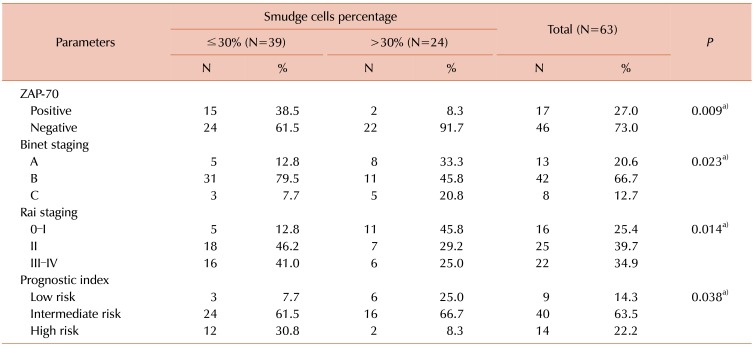
When the expression of ZAP-70 was determined at a cutoff value of 20%, 27.0% of the studied patients were considered ZAP-70 positive and 73.0% were considered ZAP-70 negative. Among the studied CLL patients; a higher percentage of SCs (>30%) was statistically significantly associated with ZAP-70 negative patients (91.7%), stage B and A Binet (45.8% and 33.3% respectively), early Rai stage (0-I and II) (45.8% and 29.2% respectively) and intermediate or low risk CLL prognostic index (66.7% and 25.0% respectively) (Table 2).
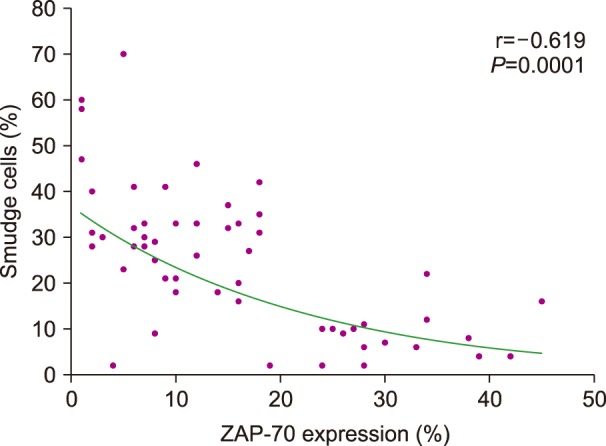
In a correlation test between the percentage of SCs and hematological parameters, ZAP-70, β2 microglobulin levels, and LDH, SC percentage was significantly negatively correlated with the percentage of ZAP-70 expression (r=-0.619, P=0.0001) (Fig. 1). There was no significant correlation with the other hematological parameters or serum markers.
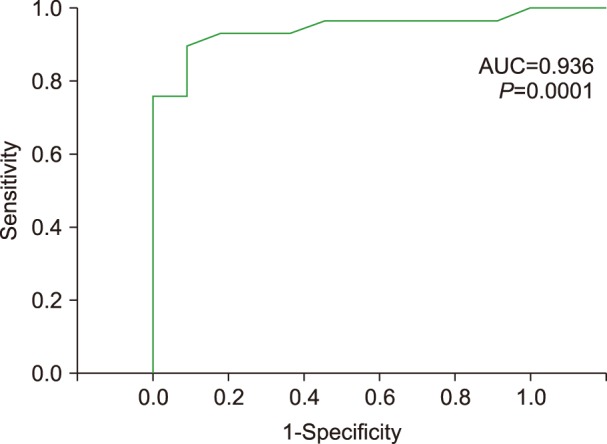
The ROC curve plotted to evaluate the value of SC percentage for prediction of positive ZAP-70 expression revealed a highly significant area under the curve of 0.936 (P=0.0001) with a cutoff value of 8.5% and sensitivity 0.966 (Fig. 2).
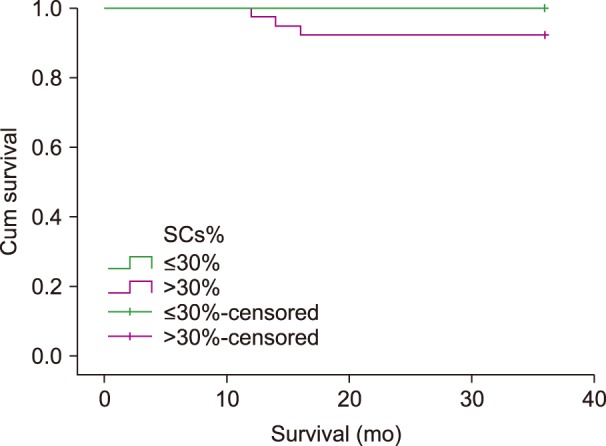
Survival analysis revealed that patients with SC percentage of >30% showed 100% overall survival at 36 months of follow up, while those with SC percentage of ≤30% showed a lower percentage of overall survival (92.3%). This difference was statistically insignificant (P=0.169) (Fig. 3).
Being common artifacts, SCs may result from any white cell in the PBF. It is attributed to traumatic disruption of the cells during blood film preparation which depends on the physical interaction during blood film smear that occurs between the cells and the glass of the slide [1516].
Usually, normal PBFs comprise 0 to 1% SCs with some studies reporting the mean proportion to be 5% (range, 0–12%). This value invariably increases to greater than 10% and sometimes greater than 50% or more than 100 SCs per 100 leukocytes in CLL patients [51718]. The present study found a wide range of SC percentage, from 2 to 58% with a mean proportion of 24.03±13.98%.
Upon rupturing the cell wall, the remaining nuclear remnants of lymphocytes are structureless chromatin material [17]. Since these cells were intact cells, both within the circulation and in the blood sample, they are counted as whole cells in the automated leukocyte count [19]. This is why the studied patients showed higher absolute lymphocytic counts.
In the study conducted by Nowakowski et al. [20], the authors observed that the formation of SCs is related to the content of a cytoskeletal protein called vimentin. Vimentin is present in leukemic cells; it is a filament protein critical for lymphocytic rigidity and integrity. Nowakowski et al. [20] discovered that an increase in vimentin content resulted in a decrease in SC percentage. This lower SC percentage was associated with a shortened time to initial therapy in early-stage CLL.
The present study found that higher percentage of SCs (>30%) was statistically significantly associated with parameters of better prognosis in CLL patients. These parameters included negative ZAP-70 expression and early clinical stage disease as estimated by the Rai and Binet staging systems, as well as the CLL prognostic index which showed lower or intermediate risk to be associated with a higher percentage of SCs >30%.
Sall et al. [21] reported findings similar to those of the present study in 2015; they identified that a low percentage of SCs (<30%) was associated with high lymphocyte count and advanced Binet stage. Previous studies reported that patients with >30% of SCs in the PBF were more likely to have a mutated IgVH pattern and, therefore, a longer time to treatment and better survival [1422]. The median overall survival depends on the stage at diagnosis, which was 10–12 years for early stages (Rai 0-I, Binet A), 7 years for stage (Rai II, Binet B) and 1.5–4 years for advanced stages (Rai III–IV, Binet C) [23].
Survival analysis in the present study was limited to 36 months; this is a short median follow-up time for CLL disease. In spite of the short duration of follow-up, it was found that the lower percentage of SCs was associated with lower overall survival over this 36-month follow-up period. This finding was statistically insignificant (P>0.05), which was contradictory to that reported in 2009 by Nowakowski et al. [24] that the percentage of SCs on blood smear was an independent predictor of overall survival among patients with CLL.
In the study conducted by Gogia et al. [22] in 2014, the authors reported that patients with <30% SCs had a shorter median progression-free period of 30 months compared with patients who had more than 30% SCs (45 mo, P=0.01). They concluded that this simple and inexpensive detection of SCs on routine blood smears seems useful in predicting progression-free and overall survival in CLL patients and might be beneficial in countries with limited resources. This finding is in accordance with that reported in the present study, with better overall survival associated with higher percentage of SCs in PBF at presentation of patients.
In our case, it was difficult to perform the IgVH mutation test, so it was agreed to depend on the surrogate marker for IgVH mutation, which is ZAP-70 expression [25]. The proportion of ZAP-70 expression in this study ranged from 1 to 45% with a mean of 16.0±12.2%. With a cut off value of 20%, the proportion of positive ZAP-70 expression was 27% and negative ZAP-70 expression was seen in 73% of participants.
In this study, the SC percentage was tested as a surrogate marker for ZAP-70 expression in order to facilitate its use in situations where flow cytometric evaluation of ZAP-70 is not easily accessible. The findings of the present study showed that SC percentage in the PBF of newly diagnosed CLL patients could be used as a surrogate marker for ZAP-70 expression since there is significant negative correlation between them. The higher the SC percentage, the lower the ZAP-70 expression, and the better the CLL prognosis. Moreover, the SC percentage was a significant predictor of ZAP-70 expression by the ROC curve analysis, which adds to the value of SC percentage in evaluating the prognoses of CLL patients and the possibility of using it as a surrogate marker for ZAP-70 expression.
The important limitation in this study is the duration of follow-up for the studied CLL patients, which was 36 months and considered shorter than that of previous studies.
This study concluded that the percentage of SCs at presentation in newly diagnosed CLL patients could be used as a surrogate marker for ZAP-70 expression and an additional prognostic marker for disease progression and recommends its estimation in low resource areas where flow cytometry is not available. However, SC counting is a manual process; in order to standardize this process, it should be performed by two pathologists with a total of 200 cell counts per slide.
References
1. Montserrat E, Hillmen P. Postgraduate haematology. In : Hoffbrand AV, Higgs DR, Keeling DM, Mehta AB, editors. Chronic lymphocytic leukaemia and other B-cell disorders. 7th ed. Oxford, UK: Wiley Blackwell;2016. p. 500–523.
2. Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of chronic (mature) B and T lymphoid leukaemias. French-American-British (FAB) Cooperative Group. J Clin Pathol. 1989; 42:567–584. PMID: 2738163.

3. McMullin MF. 70 years of the JCP-highly cited papers: Proposals for the classification of chronic (mature) B and T lymphoid leukaemias. J Clin Pathol. 2017; 70:909–910. PMID: 29070652.

4. Nosanchuk JS. The effect of smudge cells on leukocyte counting-are chamber counts necessary? Am J Clin Pathol. 1979; 71:161–166. PMID: 425933.
5. Macdonald D, Richardson H, Raby A. Practice guidelines on the reporting of smudge cells in the white blood cell differential count. Arch Pathol Lab Med. 2003; 127:105. PMID: 12562274.

6. Hematology Committee, Quality Management Program-Laboratory Services. Good practice guidelines: reporting of smudge cells. Hematology morphology binder. Toronto, Canada: Hematology-Morphology Binder;2001.
7. Johansson P, Eisele L, Klein-Hitpass L, et al. Percentage of smudge cells determined on routine blood smears is a novel prognostic factor in chronic lymphocytic leukemia. Leuk Res. 2010; 34:892–898. PMID: 20353875.

8. Binet JL, Auquier A, Dighiero G, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981; 48:198–206. PMID: 7237385.

9. Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975; 46:219–234. PMID: 1139039.

10. Gentile M, Mauro FR, Rossi D, et al. Italian external and multicentric validation of the MD Anderson Cancer Center nomogram and prognostic index for chronic lymphocytic leukaemia patients: analysis of 1502 cases. Br J Haematol. 2014; 167:224–232. PMID: 25041609.

11. Preobrazhensky SN, Bahler DW. Optimization of flow cytometric measurement of ZAP-70 in chronic lymphocytic leukemia. Cytometry B Clin Cytom. 2008; 74:118–127. PMID: 17948236.

12. Matutes E, Owusu-Ankomah K, Morilla R, et al. The immunological profile of B-cell disorders and proposal of a scoring system for the diagnosis of CLL. Leukemia. 1994; 8:1640–1645. PMID: 7523797.
13. Moreau EJ, Matutes E, A'Hern RP, et al. Improvement of the chronic lymphocytic leukemia scoring system with the monoclonal antibody SN8 (CD79b). Am J Clin Pathol. 1997; 108:378–382. PMID: 9322589.
14. Nowakowski GS, Hoyer JD, Shanafelt TD, et al. Using smudge cells on routine blood smears to predict clinical outcome in chronic lymphocytic leukemia: a universally available prognostic test. Mayo Clin Proc. 2007; 82:449–453. PMID: 17418074.

15. Allison RW, Meinkoth JH. Hematology without the numbers: in-clinic blood film evaluation. Vet Clin North Am Small Anim Pract. 2007; 37:245–266. PMID: 17336674.

16. Johnston A, McFarlane A, Bourner G, Martin T, Padmore R. Distinguishing morphology of reactive versus abnormal neoplastic peripheral blood lymphocytosis. Challenges illustrated by two proficiency testing surveys. Int J Lab Hematol. 2016; 38:e41–e44. PMID: 26872147.

17. Palmer L, Briggs C, McFadden S, et al. ICSH recommendations for the standardization of nomenclature and grading of peripheral blood cell morphological features. Int J Lab Hematol. 2015; 37:287–303. PMID: 25728865.

18. Vives-Corrons JL, Briggs C, Simon-Lopez R, et al. Effect of EDTA-anticoagulated whole blood storage on cell morphology examination. A need for standardization. Int J Lab Hematol. 2014; 36:222–226. PMID: 24330572.

19. Jacob EA. Complete blood cell count and peripheral blood film, its significant in laboratory medicine: a review study. Am J Lab Med. 2016; 1:34–57.
20. Nowakowski GS, Lee YK, Bone ND, et al. Proteomic analysis of chronic lymphocytic leukemia cells identifies vimentin as a novel prognostic factor for aggressive disease. Blood. 2005; 106:209–213.

21. Sall A, Touré AO, Sall FB, et al. Using smudge cells percentage on routine blood smear in chronic lymphoytic leukemia as prognostic factor: senegalese experience. Blood. 2015; 126:5273.

22. Gogia A, Raina V, Gupta R, et al. Prognostic and predictive significance of smudge cell percentage on routine blood smear in chronic lymphocytic leukemia. Clin Lymphoma Myeloma Leuk. 2014; 14:514–517. PMID: 24656596.

23. Hernández JA, González M, Hernández JM. Chronic lymphocytic leukemia. In : Oppezzo P, editor. Prognostic factors in chronic lymphoid leukemia and identification of new clinically relevant molecular markers. Rijeka, Croatia: InTech;2012. p. 251–268.
24. Nowakowski GS, Hoyer JD, Shanafelt TD, et al. Percentage of smudge cells on routine blood smear predicts survival in chronic lymphocytic leukemia. J Clin Oncol. 2009; 27:1844–1849. PMID: 19255329.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download