Abstract
Background
Extranodal NK/T-cell lymphoma, nasal type (ENKTCL) has a high prevalence in Asia and Latin American countries, such as Mexico, where it encompasses 40% of all T-cell non-Hodgkin lymphomas. Historically, responses to anthracycline-based therapies have been disappointing. Since data about the effectiveness of L-asparaginase-based regimens in Mexico are limited, we compared both therapies in our center.
Methods
We performed a retrospective cohort of patients with newly diagnosed ENKTCL, who were divided into two groups for treatment and analysis (group 1: L-asparaginase-based regimen and group 2: anthracycline-based regimen) between 2001 and 2016.
Results
Of 36 patients with newly-diagnosed ENKTCL, 33 received at least one cycle of chemotherapy (22 in group 1 and 11 in group 2). Over a median follow-up interval of 17 months (range, 0–167), a complete response (CR) was observed in 45.5% of patients in group 1, compared to 27% of group 2 (P=0.45). Progression was more frequently observed in group 2 than in group 1 (54.5% vs. 18.4%, P=0.04). The median overall survival (OS) was 44 months in group 1, compared to 5 months in group 2 (P=0.012). The multivariate analysis showed that failure to achieve a CR after first-line therapy was the only significant factor for OS (HR, 3.04; 95% CI, 1.4–6.5; P=0.005).
T-cell non-Hodgkin lymphoma (T-cell NHL) is estimated to account for - 10% of all non-Hodgkin lymphoma cases. The extranodal NK/T-cell lymphoma nasal type (ENKTCL), which is a T-cell NHL subtype, is an aggressive lymphoid neoplasm that usually involves the nasal and paranasal areas and is associated with poor prognosis. The average incidence of ENKTCL in the United States, Canada, and Europe is estimated to be around 5% of all T-cell NHL cases; while in Asia and in some Latin American countries, ENKTCL involves 22% of all cases [123]. Remarkably, a recent report showed that in the Mexican population, about 40% of all T-cell NHL cases are ENKTCL [3].
Historically, early stage and high-risk patients with ENKTCL have been treated with anthracycline-based therapies such as cyclophosphamide, adriamycin, vincristine, and prednisone with or without radiotherapy (CHOP+RT). Unfortunately, treatment responses have been disappointing in these patients. Early stage patients with ENKTCL who received CHOP+RT had an overall response (OR) rates between 58 and 87%, with three and five-year overall survivals (OSs) of 59 and 75%, respectively. Furthermore, these patients had a relapse probability that ranged from 28 to 57% [456]. In addition, high risk patients exhibited an OR between 20 and 40% with a 5-year OS of 30% and a probability of relapse between 30 and 70% [678].
The low response rates that have been observed with anthracycline-based regimens are due to the overexpression of the multidrug resistance (MDR1) gene product, P-glycoprotein, which effluxes anthracyclines out of cells. Importantly, this has not been observed with other compounds, such as L-asparaginase [9]. One decade ago, a study performed by Yong et al. showed for the first time that patients with relapsed/refractory ENKTCL who were treated with L-asparaginase-based regimens had good response rates. Based on this study, L-asparaginase was recommended for addition to chemotherapy regimens for this group of patients [1011].
There are many L-asparaginase-based regimens being used nowadays, with better results. Recent studies have reported OR rates between 77 and 83% and a 5-year OS between 55 and 67% for patients with relapsed/refractory ENKTCL [10111213]. Although these results suggest that the addition of L-asparaginase to first line chemotherapy regimens for patients with ENKTCL might facilitate survival improvements, no prospective phase III clinical trials have been performed to test which regimen is superior.
In Mexico, studies that have evaluated the efficacy of first-line anthracycline-based regimens and others regimens did not include anthracyclines nor L-asparaginase in patients with ENKTCL. However, their results showed complete response (CR) rates between 65 and 93% and 5-year OS between 50 and 80% [141516]. No published studies in Mexico have evaluated L-asparaginase-based therapies in patients with ENKTCL. Therefore, we performed a retrospective, single institution study to compare the efficacy of anthracycline-based regimens to L-asparaginase-based regimens that were given as first-line therapy in newly diagnosed patients with ENKTCL. The secondary objectives of our study were to estimate OS and determine the relapse and all-cause mortality rates.
The present study was conducted at the ‘Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán’ (INCMNSZ) in Mexico City, with patients who were registered from 2001 to 2016. The protocol was approved by our institutional review board. All of the patients who were analyzed in this study had de novo ENKTCL and received first-line therapy at INCMNSZ. Diagnostic criteria for ENKTCL was based on the WHO 2008 standard [17].
Patients were categorized in two groups according to the first-line therapy that they received: group 1 (L-asparaginase based therapy, protocols used in our institution since 2010) and group 2 (anthracycline based therapy, protocols prior to 2010).
Group 1 consisted of patients who were treated with two regimens: 1) LVP (L-asparaginase 6,000 IU/m2 intravenously from day 1 to 5; vincristine 1.4 mg/m2 to a maximum of 2 mg on day 1; prednisone 100 mg/day from day 1 to 5) with ‘sandwich’ radiotherapy between four cycles for Ann Arbor stage I; and 2) L-aspMetDex (L-asparaginase 6,000 IU/m2 intramuscularly, unless contraindicated, on days 2, 4, 6, and 8; methotrexate 3 g/m2 on day 1 with alkaline hydration and leucovorin rescue; dexamethasone 40 mg intravenously from days 1 to 4 with ‘sandwich radiotherapy’ between four cycles for Ann Arbor stage II, and a total of six cycles without radiotherapy for patients with advanced-stage ENKTCL [18]. Fibrinogen levels were measured before every cycle and every 48 hours, once therapy was initiated. Patients with fibrinogen levels below 100 mg/dL received replacement therapy [19].
Patients in group 2 were treated with two regimens: 1) CHOP (cyclophosphamide 750 mg/m2 intravenously on day 1; doxorubicin 50 mg/m2 on day 1; vincristine 1.4 mg/m2 to a maximum of 2 mg on day 1; prednisone 100 mg/day from day 1 to 5), for six cycles, and 2) CHOEP (cyclophosphamide 750 mg/m2 intravenously on day 1; doxorubicin 50 mg/m2 on day 1; vincristine 1.4 mg/m2 to a maximum of 2 mg on day 1; prednisone 100 mg/day from day 1 to 5; etoposide 100 mg/m2 on days 1 to 3) for six cycles [2021]. Both regimens and radiotherapy were used for patients with stage I or II disease and were offered according to medical criteria in advance disease scenarios.
The pre-treatment evaluations included clinical history and physical examination, serum biochemistry, complete blood count, lactate dehydrogenase (LDH), bone marrow biopsy, computed tomography of neck, chest, abdomen, and pelvis, and magnetic resonance imaging of the head and neck.
Baseline clinical characteristics of patients with ENKTCL that were analysed in our study included: age, gender, ECOG performance status (Eastern Cooperative Oncology Group), LDH, Ann Arbor stage, International Prognostic Index (IPI), prognostic index for ENKTCL (NKPI), and since the newest prognostic index, PINK, is applicable only for patients not receiving antraclyclines, we only measured PINK in patients treated with L-asparaginase-based regimens in our cohort [222324]. The final response to treatment was evaluated after four and/or six cycles, depending on the stage of the illness. This was performed according to the Lugano classification [25].
Statistical significance was determined by the Mann Whitney test for continuous variables and for categorical variables, the Chi squared and Fisher exact tests were employed. A Kaplan-Meier analysis was used to estimate OS and Cox regression models, which were employed to identify the significant prognostic factors for OS. A P-value of <0.05 was considered statistically significant.
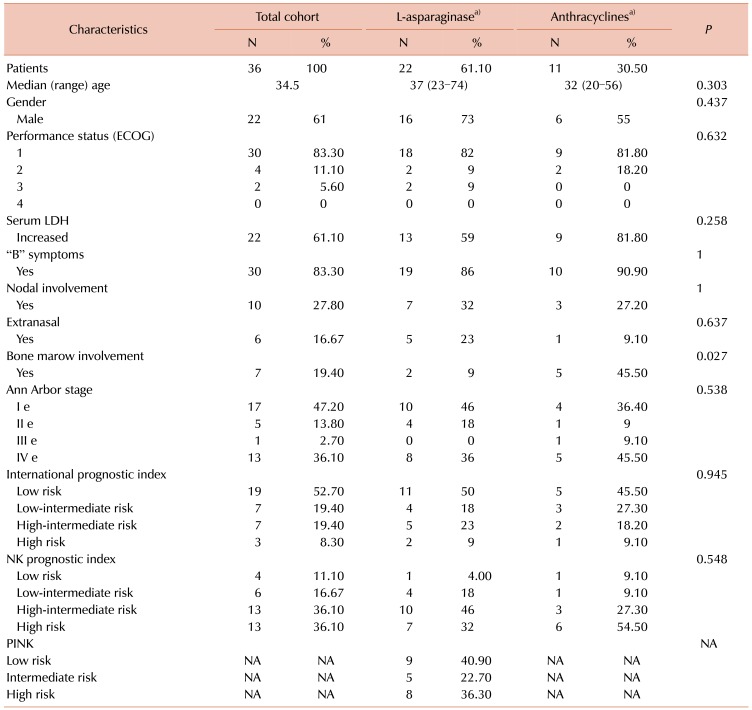
Overall, 36 patients were included in the clinical and demographic analysis and the patient characteristics are presented in Table 1. Three patients were excluded from the outcomes and survival analyses because they were treated with a different regimen than those indicated above. Thirty-three patients received at least one cycle of chemotherapy, 22 in group 1 and 11 in group 2. In group 1, eight patients received LVP regimens and fourteen patients received LaspMetDex. In group 2, seven patients received the CHOP regimen and four received CHOEP. Eighteen patients (54.5%) received radiotherapy as part of their treatment protocol; nineteen patients had stage I or II diseases, of whom 15 (78.9%) received radiotherapy: six patients received LVP, six received LaspMetDex, two received CHOP, and one received CHOEP. Only three patients with advanced disease standard protocol received radiotherapy, based on their medical criteria and clinical scenarios: two in group 1 and one in group 2.
Only six patients (18.2%) received high dose chemotherapy followed by autologous stem cell transplantation: five in group 1 (22.7%) and one in group 2. There were no statistically significant differences between groups regarding basal characteristics, except for bone marrow involvement, which was more frequent in group 2 (9% vs. 45%, P=0.027).
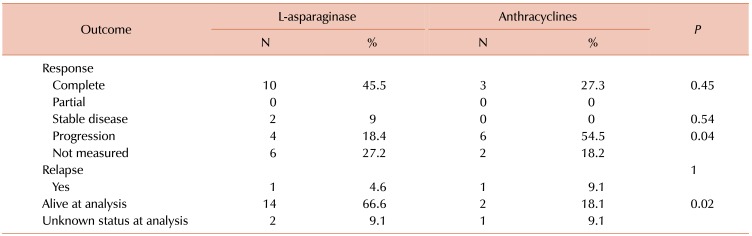
CR rates were greater in group 1 than in group 2 (45.5% vs. 27.3%), as presented in Table 2. However, statistical significance was not achieved (P=0.45). According to specific treatment in group 1, of the eight patients who received LVP regimens, five (64.3%) reached CR, while of the 13 patients who received L-aspMetDex regimens, five (35.7%) reached CR (P=0.37). In group-2, two (28.57%) of the patients on CHOP and one (25%) on CHOEP reached CR (P=0.72).
In a sub-analysis that was performed for the fifteen patients with stage I and II ENKTCL who received radiotherapy, 11 (73%) reached CR, compared to none of the patients who did not receive radiotherapy (P=0.018).
As shown in Table 2, progression was more frequently observed in group 2 than in group 1 (54.5% vs. 18.4%, P=0.04), and according to specific treatment, in group 1, progression was observed in 25% of patients on LVP regimens and in 15.38% of patients on LaspMetDex (P=0.68). In group 2, progression was observed in three (42%) patients on CHOP and three (75%) patients on CHOEP regimens (P=0.54).
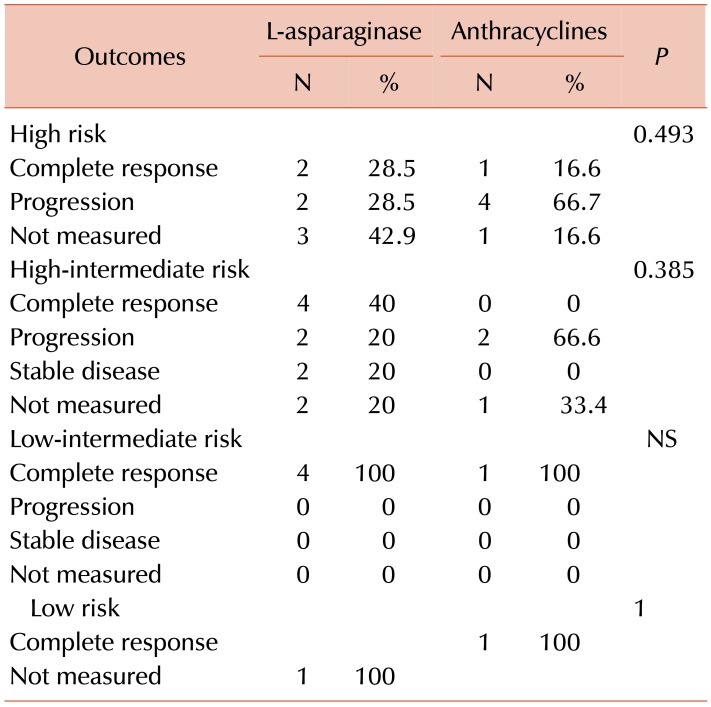
In group 1, there were no deaths in the LVP arm. In the patients on LaspMetDex there was a mortality rate of 42.85%: four (66.6%) of these patients had advanced stage disease. In group 2, five (71%) patients on CHOP regimens and three (75%) patients on CHOEP died. As shown in Table 3, patients from group 1 showed a higher CR rate, which was independent of clinical stage for NKPI. All five patients with stage IV ENKTCL in group 2 died.
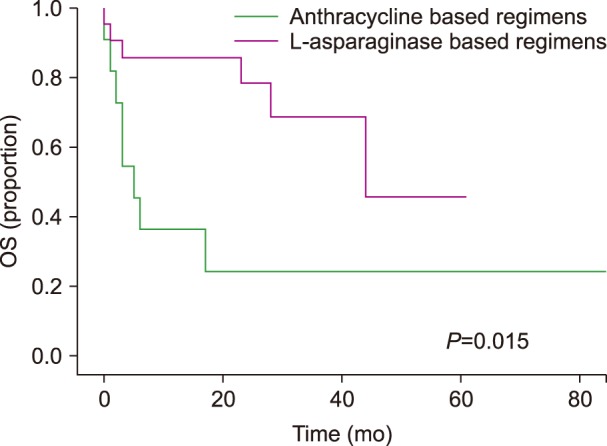
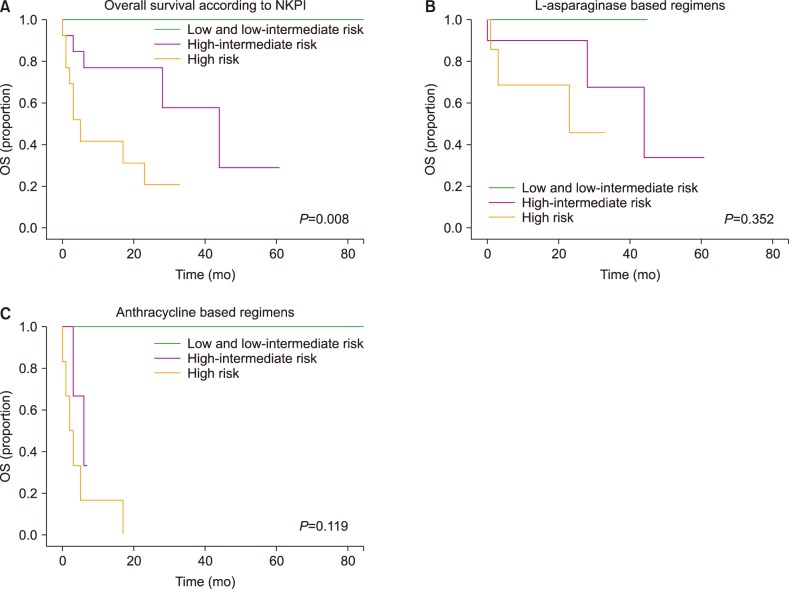
At the time of analysis, there was a higher percentage of patients who were alive in group 1 than in group 2 (66.6% vs. 18.1%; P=0.02), as shown in Table 2. Over a median follow-up interval of 17 months (range, 0–167), the median OS was 44 months. The OS was 44 months for group 1 and 5 months for group 2 (P=0.015), as presented in Fig. 1. The median OS was not reached for patients with early stage disease, irrespective of whether they received anthracyclines or an L-asparaginase-based regimens (P=0.363). Patients with early stage disease who received radiotherapy did not reach the median OS, compared to the OS of six months that was observed in patients who did not receive radiotherapy (P=0.001). In the entire group, the 5-year OS according to NKPI was 100% for low and low intermediate risk, 28% for high-intermediate risk, and 20% for high risk patients (P=0.008).
In group 1, the 5-year OS according to NKPI was 100% for low-intermediate risk, 33% for high-intermediate risk, and 45% for high risk patients, while in group 2, the rate was 100% for low-intermediate risk and 0% for high and high-intermediate risk patients, as shown in Fig. 2.
Of the patients who received high dose chemotherapy followed by autologous stem cell transplantation, the median overall survival was not reached, compared to a median OS of 28 months that was observed for patients who did not receive autologous stem cell transplantation.
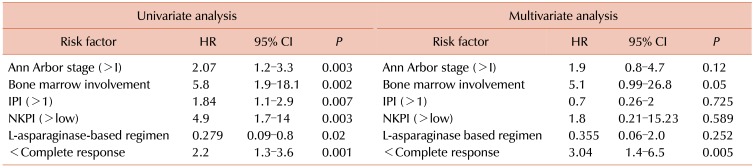
The univariate analysis revealed six risk factors for OS. Interestingly, treatment with an L-asparaginase based regimen was a protective factor (HR, 0.279; 95% CI, 0.09–0.8; P=0.02), as listed in Table 4.
The multivariate analysis showed that not reaching a complete remission was the only independent adverse risk factor for OS (HR, 3.04; 95% CI, 1.4–6.5; P=0.005).
In a sub-analysis for overall survival that was performed for the early stage disease group, the only risk factor for OS that was found in the univariate analysis was radiotherapy, which was a protective factor (HR, 0.059; 95% CI, 0.006–0.57; P=0.015).
To our knowledge, this is the first study in the Mexican population to compare the effectiveness of L-asparaginase-based regimens as first line therapy to previously-offered anthracycline regimens in patients with ENKTCL, across all clinical stages.
There is limited data on the effectiveness of L-asparaginase-based regimens in non-Asian populations, such as Latin Americans. In 2016, Qi et al. published a retrospective study of 43 patients with ENKTCL in North America, which consisted of 76% non-Asians and 23% Asians. Patients with early-stage disease (73%) received chemotherapy followed by radiotherapy, and patients with advanced-stage disease received primary chemotherapy (accelerated-CHOP prior to 2009 and modified-SMILE after 2009). CR rates were reached in a higher percentage of patients who received modified-SMILE regimens (80% vs. 30%, P=0.015), with a 2-year OS and progression free survival of 60% and 40% respectively. However in agreement with other published data, the outcomes of patients with early stage disease were better than those with advanced stage disease, who experienced frequent progression and high mortality. So far, no prognostic difference have been identified in terms of ethnicity [26].
Another recent retrospective study from Huang et al. [27] compared the efficacy between the “sandwich” LVD regimen and the ‘sandwich’ CHOP regimen in 80 Asiatic patients with stage II ENKTCL. They found a statistical difference between objective response rates in patients treated with LVD (89.6%) and CHOP (65.6%; P=0.009). Moreover, they reported disease progression in 8.3% and 31.3% of patients who were treated with LVD and CHOP, respectively. A significant advantage in OS and progression free survival was also observed. Importantly, experts have ceased to recommend the inclusion of anthracyclines to ENKTCL treatment schemes, based on the fact that this tumour shows multidrug resistance due to P-glycoprotein overexpression and the disappointing responses to anthracycline-based regimens in previously published clinical studies [9].
Interestingly our data showed a lower median age for patients in our cohort (35.4 yr) compared to those in other studies (44–52 yr) that included asian and non-asian individuals [132628]. However, despite their young age, it seems that they do not tolerate chemotherapy as well as those in other latitudes, as reflected by our high early mortality. In agreement with other reports, we found a predominance of ENKTCL in the male population. In agreement with previous reports that showed a low efficacy for anthracycline-based regimens in patients with ENKTCL, our data showed a global CR rate of 27.3% (50% in stage I, 0% in stage II, and 20% in stage IV) [29]. This whole group showed a 5-year OS of 24%.
In contrast, patients treated with L-asparaginase showed a CR rate of 45.4% (70% in stage I, 50% in stage II and 12.5% in stage IV), with a 5-year OS of 45%. This represents a higher response rate than that observed for other anthracycline-based regimens, although the difference in CR rates between both groups was not statistically significant, probably due to the relatively small number of patients analyzed. The all-cause mortality was 27.3% in group 1 versus 72% in group 2 (P=0.027). Moreover, the L-asparaginase-treated group showed a lower progression rate (18.4 vs. 54.5%; P=0.04) than the anthracycline treated group. The relapse rate could not be measured because only one patient in each group showed relapse. Interestingly, our results showed that LVP-treated patients with stage I disease had an CR rate of 64.3%, while those with stage II to IV disease who were treated with L-aspMetDex had a CR rate of 35.7%. These values are significantly lower than those reported by Huang and other studies for similar groups of patients [1819].
The use of L-asparaginase as a cytotoxic agent for the treatment of relapsed/refractory disease was first introduced 10 years ago, with good results [10111213]. However, there remains some controversy about which chemotherapeutic regimen is the most adequate as first-line therapy for patients with ENKTCL. Regimens such as SMILE (dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide) have been studied in newly diagnosed patients across all clinical stages and in relapsed/refractory scenarios. SMILE regimens have achieved OR rates of almost 80%, complete responses between 45 and 56%, and an OS of 50%, but are also associated with high-grade 3 toxicities [3031]. Particularly, the incorporation of the SMILE regimen into our institution treatment protocols has not been possible because of its high costs. For example, the L-aspMetDex regimen could cost around $867 per cycle, while the SMILE regimen is around $1,694, without the cost of granulocyte colony stimulating factor (G-CSF) included in the regimen protocol. This chemotherapeutic protocol is not currently covered by any social security system. The same situation is present in the transplantation protocols of our institution, where the social security systems of our country just began to cover this procedure. Therefore, in our cohort, many patients could not receive this treatment modality, which can potentially change the course of the disease, based on response and risk factors.
The impact of radiotherapy is also confirmed by our results. No matter which chemotherapeutic regimen was offered, the combined modality is of great use in this particular type of lymphoma in terms of response rates and survival in early stages [561827].
It is possible that the true impact of L-asparaginase in first line therapy for ENKTCL could not be fully assessed in our population, due to the low number of patients who were included in this retrospective study and because responses could not be measured in eight (24%) patients, six of whom died early, during the first two cycles of chemotherapy, due to septic shock. The majority of these patients were treated with LaspMetDex regimens, one patient abandoned treatment, and another was still on treatment at the time of our analysis.
Although our study is restrospective and has a limited sample size, our data showed an evident survival advantage for patients with ENKTCL who were treated with L-asparaginase-based regimens in comparison to those treated with anthracycline-based regimens. Due to the higher incidence of ENKTCL in the Mexican population, in contrast to other geographical regions, we propose that the inclusion of L-asparaginase should be considered in treatments across all clinical stages. However, we are still in need of a larger study to validate our results and identify the factors that impact outcomes across latitudes, such as ancestry-specific factors, differences in treatment adherence, and access to therapy.
ACKNOWLEDGMENTS
The Authors thank the medical staff of the Department of Hematology-Oncology of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán.
References
1. Vose J, Armitage J, Weisenburger D. International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008; 26:4124–4130. PMID: 18626005.
2. Laurini JA, Perry AM, Boilesen E, et al. Classification of non-Hodgkin lymphoma in Central and South America: a review of 1028 cases. Blood. 2012; 120:4795–4801. PMID: 23086753.

3. Avilés A. Nasal NK/T-cell lymphoma. A comparative analysis of a mexican population with the other populations of Latin-America. Mediterr J Hematol Infect Dis. 2015; 7:e2015052. PMID: 26401241.
4. Kim WS, Song SY, Ahn YC, et al. CHOP followed by involved field radiation: is it optimal for localized nasal natural killer/T-cell lymphoma? Ann Oncol. 2001; 12:349–352. PMID: 11332147.

5. Li YX, Yao B, Jin J, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol. 2006; 24:181–189. PMID: 16382127.

6. Ma HH, Qian LT, Pan HF, et al. Treatment outcome of radiotherapy alone versus radiochemotherapy in early stage nasal natural killer/T-cell lymphoma. Med Oncol. 2010; 27:798–806. PMID: 19685292.

7. Cheung MM, Chan JK, Lau WH, et al. Primary non-Hodgkin's lymphoma of the nose and nasopharynx: clinical features, tumor immunophenotype, and treatment outcome in 113 patients. J Clin Oncol. 1998; 16:70–77. PMID: 9440725.

8. Chim CS, Ma SY, Au WY, et al. Primary nasal natural killer cell lymphoma: long-term treatment outcome and relationship with the International Prognostic Index. Blood. 2004; 103:216–221. PMID: 12933580.

9. Yamaguchi M, Kita K, Miwa H, et al. Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer. 1995; 76:2351–2356. PMID: 8635042.

11. Yong W, Zheng W, Zhang Y, et al. L-asparaginase-based regimen in the treatment of refractory midline nasal/nasal-type T/NK-cell lymphoma. Int J Hematol. 2003; 78:163–167. PMID: 12953813.

12. Yong W, Zheng W, Zhu J, et al. L-asparaginase in the treatment of refractory and relapsed extranodal NK/T-cell lymphoma, nasal type. Ann Hematol. 2009; 88:647–652. PMID: 19107482.

13. Tse E, Kwong YL. Diagnosis and management of extranodal NK/T cell lymphoma nasal type. Expert Rev Hematol. 2016; 9:861–871. PMID: 27347812.

14. Sobrevilla-Calvo P, Meneses A, Alfaro P, Bares JP, Amador J, Reynoso EE. Radiotherapy compared to chemotherapy as initial treatment of angiocentric centrofacial lymphoma (polymorphic reticulosis). Acta Oncol. 1993; 32:69–72. PMID: 8466767.

15. Avilés A, Díaz NR, Neri N, Cleto S, Talavera A. Angiocentric nasal T/natural killer cell lymphoma: a single centre study of prognostic factors in 108 patients. Clin Lab Haematol. 2000; 22:215–220. PMID: 11012633.

16. Avilés A, Neri N, Fernández R, Huerta-Guzmán J, Nambo MJ. Combined therapy in untreated patients improves outcome in nasal NK/T lymphoma: results of a clinical trial. Med Oncol. 2013; 30:637. PMID: 23797771.

17. Chan J, Quintanilla-Martínez L, Ferry J, Peh S. Extranodal NK/T cell lymphoma, nasal type. In : Swerdlow SH, Campo E, Harris NL, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC Press;2008. p. 285–288.
18. Jiang M, Zhang H, Jiang Y, et al. Phase 2 trial of “sandwich” L-asparaginase, vincristine, and prednisone chemotherapy with radiotherapy in newly diagnosed, stage IE to IIE, nasal type, extranodal natural killer/T-cell lymphoma. Cancer. 2012; 118:3294–3301. PMID: 22139825.
19. Jaccard A, Gachard N, Marin B, et al. Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood. 2011; 117:1834–1839. PMID: 21123825.

20. McKelvey EM, Gottlieb JA, Wilson HE, et al. Hydroxyldaunomycin (Adriamycin) combination chemotherapy in malignant lymphoma. Cancer. 1976; 38:1484–1493. PMID: 791473.

21. Pfreundschuh M, Trümper L, Kloess M, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood. 2004; 104:634–641. PMID: 15016643.

22. International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993; 329:987–994. PMID: 8141877.
23. Lee J, Suh C, Park YH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol. 2006; 24:612–618. PMID: 16380410.

24. Kim SJ, Yoon DH, Jaccard A, et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol. 2016; 17:389–400. PMID: 26873565.

25. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014; 32:3059–3068. PMID: 25113753.

26. Qi S, Yahalom J, Hsu M, et al. Encouraging experience in the treatment of nasal type extra-nodal NK/T-cell lymphoma in a non-Asian population. Leuk Lymphoma. 2016; 57:2575–2583. PMID: 27183991.

27. Huang L, Yuan B, Wu H, et al. Comparative study of L-asparaginase-based LOP regimen over CHOP regimen before radiotherapy for stage IIE extranodal nasal type NK/T cell lymphoma: a study of 2 centers. Clin Lymphoma Myeloma Leuk. 2017; 17:152–158. PMID: 28215935.

28. Au WY, Weisenburger DD, Intragumtornchai T, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009; 113:3931–3937. PMID: 19029440.

29. Makita S, Tobinai K. Clinical features and current optimal management of natural killer/T-cell lymphoma. Hematol Oncol Clin North Am. 2017; 31:239–253. PMID: 28340876.

30. Yamaguchi M, Kwong YL, Kim WS, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol. 2011; 29:4410–4416. PMID: 21990393.

31. Kwong YL, Kim WS, Lim ST, et al. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood. 2012; 120:2973–2980. PMID: 22919026.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download