1. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992; 41:1–19.
2. Adami J, Gäbel H, Lindelöf B, et al. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer. 2003; 89:1221–1227. PMID:
14520450.

3. Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011; 105:93–103. PMID:
21629244.

4. Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013; 10:230–252. PMID:
23604045.

5. Woo JR, Liss MA, Muldong MT, et al. Tumor infiltrating B-cells are increased in prostate cancer tissue. J Transl Med. 2014; 12:30. PMID:
24475900.

6. Tzankov A, Meier C, Hirschmann P, Went P, Pileri SA, Dirnhofer S. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin's lymphoma. Haematologica. 2008; 93:193–200. PMID:
18223287.

7. Mociková H. Prognostic significance of absolute lymphocyte count and lymphocyte subsets in lymphomas. Prague Med Rep. 2010; 111:5–11.
8. Alcasid M, Ma L, Gotlib JR, Arber DA, Ohgami RS. The clinicopathologic significance of lymphocyte subsets in acute myeloid leukemia. Int J Lab Hematol. 2017; 39:129–136. PMID:
28133918.

9. Le Dieu R, Taussig DC, Ramsay AG, et al. Peripheral blood T cells in acute myeloid leukemia (AML) patients at diagnosis have abnormal phenotype and genotype and form defective immune synapses with AML blasts. Blood. 2009; 114:3909–3916. PMID:
19710498.

10. Stringaris K, Sekine T, Khoder A, et al. Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica. 2014; 99:836–847. PMID:
24488563.

11. Vidriales MB, Orfao A, López-Berges MC, et al. Lymphoid subsets in acute myeloid leukemias: increased number of cells with NK phenotype and normal T-cell distribution. Ann Hematol. 1993; 67:217–222. PMID:
7694663.

12. Bruserud O. Acute myelogenous leukemia blasts as accessory cells during T lymphocyte activation: possible implications for future therapeutic strategies. Leukemia. 1999; 13:1175–1187. PMID:
10450745.

13. Siegler U, Kalberer CP, Nowbakht P, Sendelov S, Meyer-Monard S, Wodnar-Filipowicz A. Activated natural killer cells from patients with acute myeloid leukemia are cytotoxic against autologous leukemic blasts in NOD/SCID mice. Leukemia. 2005; 19:2215–2222. PMID:
16224486.

14. Ohnishi K, Yamanishi H, Naito K, et al. Reconstitution of peripheral blood lymphocyte subsets in the long-term disease-free survivors of patients with acute myeloblastic leukemia. Leukemia. 1998; 12:52–58. PMID:
9436920.

15. Parrado A, Casares S, Prieto J, Carmona M, Vaquero A, Rodríguez-Fernández JM. Repopulation of circulating T, B and NK lymphocytes following bone marrow and blood stem cell transplantation. Hematol Cell Ther. 1997; 39:301–306. PMID:
9497889.

16. Bar M, Othus M, Park HM, et al. Elevated lymphocyte count at time of acute myeloid leukemia diagnosis is associated with shorter remission. Leuk Lymphoma. 2015; 56:3109–3115. PMID:
25726954.

17. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016; 127:2391–2405. PMID:
27069254.

18. Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010; 116:354–365. PMID:
20385793.

19. Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003; 21:4642–4649. PMID:
14673054.

20. Choi J, Lee SJ, Lee YA, Maeng HG, Lee JK, Kang YW. Reference values for peripheral blood lymphocyte subsets in a healthy korean population. Immune Netw. 2014; 14:289–295. PMID:
25550695.

21. Palmer S, Hanson CA, Zent CS, et al. Prognostic importance of T and NK-cells in a consecutive series of newly diagnosed patients with chronic lymphocytic leukaemia. Br J Haematol. 2008; 141:607–614. PMID:
18384436.

22. Aggarwal N, Swerdlow SH, TenEyck SP, Boyiadzis M, Felgar RE. Natural killer cell (NK) subsets and NK-like T-cell populations in acute myeloid leukemias and myelodysplastic syndromes. Cytometry B Clin Cytom. 2016; 90:349–357. PMID:
26648320.

23. Ustun C, Miller JS, Munn DH, Weisdorf DJ, Blazar BR. Regulatory T cells in acute myelogenous leukemia: is it time for immunomodulation? Blood. 2011; 118:5084–5095. PMID:
21881045.

24. Guo W, Xing C, Dong A, et al. Numbers and cytotoxicities of CD3+CD56+ T lymphocytes in peripheral blood of patients with acute myeloid leukemia and acute lymphocytic leukemia. Cancer Biol Ther. 2013; 14:916–921. PMID:
24030391.
25. Panoskaltsis N, Reid CD, Knight SC. Quantification and cytokine production of circulating lymphoid and myeloid cells in acute myelogenous leukaemia. Leukemia. 2003; 17:716–730. PMID:
12682629.

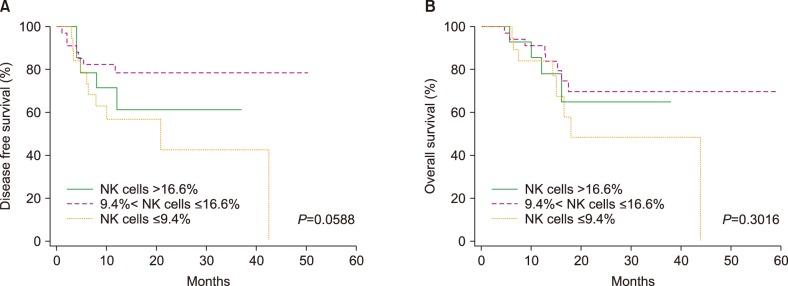
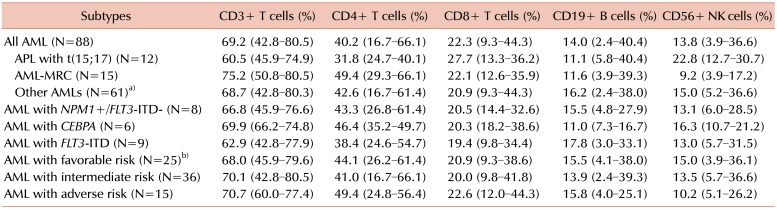
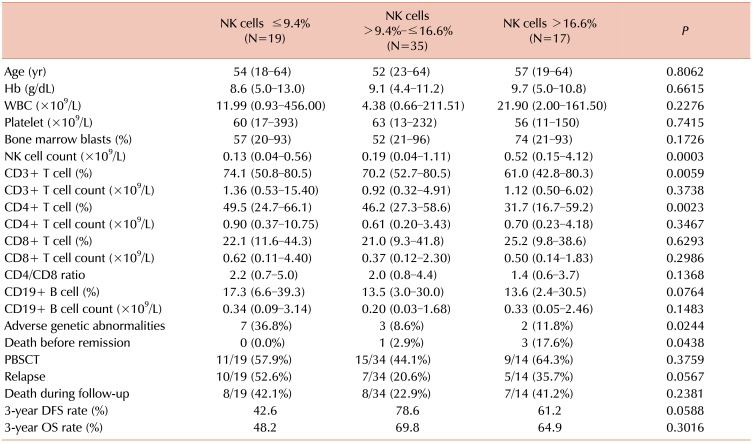
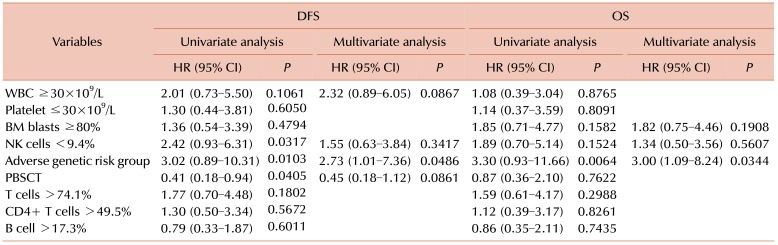




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download