Abstract
Background and Objectives
Preoperative identification of intimal tear site in acute type A dissection will help procedural planning. The objective of this study was to determine the key findings of computed tomography (CT)-based prediction for tear site and compare the accuracy between radiologists and surgeons.
Subjects and Methods
Multi-detector CT (MDCT) images from 50 patients who underwent surgical repair of type A aortic dissection were retrospectively reviewed by 4 cardiac surgeons with limited experience or by 3 radiologists specialized in cardiovascular imaging. Surgical findings of intimal tear site were used as references.
Results
In surgical findings, the locations of intimal tear that were identified in 43 patients included aorta (n=25), ascending with arch (n=7), and arch only (n=11). The rest were retrograde dissections from the tear of descending aorta. Key CT findings that were most frequently found were defect in the intimal flap shadow (30.0±4.0 patients/reviewer, accuracy 87.0±11.7%) and differential filling of false lumen by phase and location (9.4±2.9 patients/reviewer, 84.8±10.4%). Surgeons predicted tear site (75.0±7.7% vs. 86.7±1.2%, p=0.055) and specified flap defect (80.5±10.3% vs. 95.7±7.4%, p=0.073) with lower accuracy than radiologists.
Conclusions
With MDCT imaging, well-educated surgeons could be accurate in three fourths of cases. There was room for improvement through experience. Considering the substantial possibility of inaccuracy, critical decisions on CT images should be made through thorough reviewing by as many experienced radiologists and surgeons as possible.
Preoperative identification of locations and characteristics of intimal tears is important for surgical or endovascular repair of acute aortic dissection. It can be frequently achieved with high efficiency and accuracy by computed tomography (CT).1)2)3)4) Intimal tear identification is one of the most important determinants of success for endovascular repair, which is now a standard therapy for complicated type B dissection.5)6) It is sporadically performed for type A dissection with a tear in the descending7)8) or even in the ascending aorta.9)10) Although CT is considered useful in informing feasibility and treatment in the latter challenging cases,11)12) there is scarce literature and incomplete definition on its accuracy for intimal tear localization.13)14)15)16)17)
The optimal surgical strategy for acute type A dissection remains to be defined. However, intimal tear location is an important factor for determining the extent of aortic resection, perfusion strategy, and the outcome of surgery.18)19)20) Its accurate prediction may have a positive impact on the outcome of patients. Due to the emergency nature of acute dissection repair, clinical practice with only two surgeons reviewing preoperative CT images without informing radiologist is frequent. Therefore, it is important to gain insight on the accuracy of surgeons in interpreting CT information. Using surgical findings as references, this study aimed to: 1) investigate and compare radiologists' and surgeons' accuracy in predicting intimal tear sites by CT findings; and 2) determine key findings related to intimal tear location.
This retrospective observational study was approved by the Institutional Review Board with a waiver of individual patient's consent. Between January 2008 and February 2012, a total of 87 patients underwent surgical treatment for Stanford type A aortic dissection in our institution. Of them, 50 patients were enrolled for this study after using the following exclusion criteria: 1) had entirely thrombosed false lumen (intramural hematoma); 2) dissection was confined to the ascending aorta (DeBakey type II); 3) had undergone surgery more than 2 weeks after onset (chronic dissection); 4) had no soft copy data of preoperative contrast-enhanced CT images, and 5) had no description or obscure description of intimal tear in the operation record. The mean age of the 50 patients was 54.8±4.4 years (range from 27 to 84 years). Twenty-five patients were females. Eight patients had Marfan syndrome (Table 1).
The scanning equipment and technique for CT imaging varied because 27 patients underwent preoperative CT scanning at referring hospitals. Even at our own institution, three different types of multi-detector CT (MDCT) scanner were used: 16-slice (Mx8000IDT; Philips Medical Systems, Best, the Netherlands), 64-slice (Brilliance 64; Philips Medical Systems, Best, the Netherlands), and 256-slice (Brilliance iCT; Philips Medical System, Best, the Netherlands). For all images, 64-slice MDCT was the most frequently used one (25 patients), followed by 16-slice (12 patients) and 256-slice MDCT (12 patients). The other patient was examined with a single detector spiral CT scanner.
The scanning was gated with electrocardiography (ECG) in 10 patients. The majority of the cases (40 patients) were scanned with 5-mm slice thickness. Although we have a CT protocol for chest pain to rule out acute myocardial infarction, acute pulmonary embolism, or acute dissection, we do not always perform gated-CT under emergent situation because gated-CT needs controlled heart rate that can be hazardous on shock status. The entire lengths of the aorta with brachiocephalic branches and iliac arteries were scanned in 27 patients. Thoracic aorta was covered in the remaining. Multi-planar reformatting (MPR) images were available for 42 patients.
The surgical technique used in this series was uniform. The right axillary artery was preferred for arterial cannulation. Deep hypothermic circulatory arrest was used for all patients. The ascending aorta was not cross-clamped until the outer wall of the false lumen was opened. After aspirating blood from the false lumen, the intimal flap was inspected to find the tear. The ascending aorta was transected at 2-3 cm above the sinotubular junction and the interior of the ascending aorta and the aortic root were inspected. The ascending aorta was excised up to 1-2 cm below the ostium of the innominate artery. Thorough inspection was performed to find intimal tear in the transverse arch and its branches. A dentist's mirror was used to see the distal arch and proximal descending aorta as distally as possible. Retrograde and/or antegrade cerebral perfusion was started only after such systematic inspection was completed. The clinical data of all patients including operative outcomes are summarized in Table 1.
Seven clinicians participated in the reviewing of CT images to predict the site of intimal tear (Table 1). Three radiologists were specialized in cardiovascular imaging. Of the four surgeons, one is a practicing surgeon with experience of less than 30 dissection operations. The other three are trainees with experience in assisting in type A dissection repair for less than 20 cases.
All radiologists were completely blind to surgical findings. Although the surgeons had been involved in surgeries for 3 to 7 patients in this series, they were blinded to the surgical findings at the time of reviewing the CT images. The corresponding author was the main operating surgeon in this series (47 patients). Because he might know the surgical findings, the result of his review was excluded from the analysis.
The seven clinicians retrospectively and independently reviewed the CT images on a workstation or personal computer with the hospital's picture archiving and communication system (PACS Infinitt, Infinitt Co., Seoul, Korea). They obtained raw CT images without previous CT reading or patient information and summited their opinions within 7 days. For each patient, the reviewers selected what he or she judged as the site of intimal tear among 4 segments: ascending aorta, inferior arch, superior arch, or beyond arch (Fig. 1). Ascending aorta was defined as the segment below the line drawn from the innominate vein-superior vena cava junction perpendicularly to the longitudinal axis of the aorta. Inferior arch was defined as the lower half of the transverse arch. Superior arch included the proximal descending aorta above the upper margin of the main pulmonary artery. When the tear was thought to have extended along two segments, it was assigned to a more distal level.
The reviewers were required to describe the key findings or rationale for their judgments about the location of the intimal tear. To address interpersonal differences caused by indistinct demarcation in the definition of segments, the principal author unified what the reviewers answered differently based on the same key finding after examining other reviewers' judgment and rationale.
Intraoperative findings were acquired by retrospective reviewing operation records. The reviewers' judgment was validated using surgical findings as reference standards. For each reviewer, sensitivity, specificity and accuracy for finding intimal tear were calculated. The sensitivity and specificity for ascending aorta tear and arch tear were calculated separately. For patients having multiple tears located in different segments, the judgment was considered as accurate only when all surgically confirmed tears were correctly predicted. If the reviewers answered that they could not predict the intimal tear location in some patients, their answers were treated as negative for intimal tear in all segments of the aorta.
The key CT findings suggested by reviewers were validated to determine whether the judgment based on such findings was concordant with surgical findings of intimal tear location. In addition, multiple regression analyses were performed to determine the relationship between the location of intimal tear and the CT findings that were not subject to reviewers' interpretation, including the maximal diameter of the ascending aorta, thrombosis of the false lumen of the ascending aorta, the presence of intimal flap in the brachiocephalic branches, and the presence of pericardial effusion.
For statistical analysis, student's t-test was used to compare numerical values. Chi-square or Fisher's exact test was used for categorical values. Calculations were performed with SPSS 15.0 for Windows (SPSS Inc., Chicago, IL, USA). Statistical significance was considered when p was less than 0.05.
During surgery, the intimal tear was found in the surgical field of 43 (86%) patients. The remaining 7 (14%) patients were considered to have retrograde dissection from the entry tear located in the descending aorta. In 25 (50%) patients, the intimal tear was only found in the ascending aorta. Eighteen (36%) patients had a tear in the arch, 13 in the superior arch, and 5 in the inferior arch. Of them, 7 patients (14% in the series) had two separate tears in the ascending aorta and the arch. In such cases, the tears in the arch found in the superior arch around the ostia of brachiocephalic branches were always smaller than those in the ascending aorta.
The reviewers predicted the location of intimal tear in 47 patients (94%, range 42-49). They gave up prediction for an average of 3 patients. As shown in Table 1, the accuracy in the surgeons group was lower and less homogeneous than that in the radiologists group. According to intimal tear location, the sensitivity and specificity were significantly different. The sensitivity was 88.0±10.6% for ascending aorta tear vs. 62.4±12.5% for arch tear (p<0.05). The specificity was 83.3±10.2% for ascending aorta tear vs. 94.2±6.1% for arch tear (p<0.05).
The seven reviewers' judgments of intimal tear location were unanimous or consented by the majority (five or six reviewers) for 23 and 16 patients, respectively (39 patients in total, 78% of all patients). In 38 (97.4%) of the 39 cases, majority of the judgment was in agreement with the surgical findings. On the contrary, dominant interpretation was accurate in only 6 (54.5%) of the remaining 11 patients in whom the judgment was less homogeneous. The surgeons frequently made interpretations that were distinct from the majority's consensus than the radiologists (average 4.8 vs. 1.7 cases/reviewer). When only radiologists' judgments were compared, they were in consensus for 39 patients (78%), of which 37 (94.9%) were concordant with the surgical findings (Table 2).
The accuracy in predicting intimal tear location did not differ according to the following parameters related to imaging equipment and technique: site of imaging (our center vs. outside hospitals), type of scanner (16-slice or earlier vs. 64-slice vs. 256-slice MDCT), scan extent (chest CT vs. whole aorta CT), presence of MPR, slice thickness (<5 mm vs. 5 mm vs. >5 mm), and ECG-gate.
The CT finding that was the most frequently mentioned as key for intimal tear was the presence of defect (discontinuity, gap) in the intimal flap shadow (Fig. 2 and Fig. 3). The reviewers pointed out the radiological flap defect in an average of 30.0±4.0 patients. Judgment based on such finding was accurate in 87.0±11.7% of the cases. While 95.7±7.4% of the flap defects pointed out by the radiologists were concordant with the surgical findings on average, only 80.5±10.3% of what the surgeons pointed out was concordant (p=0.07). The second most useful finding was abrupt change of false lumen patency (i.e., thrombosed proximally and patent distally) at a certain point (Fig. 3). Abrupt change of false lumen patency was suggested as key in 9.4±2.9 patients/reviewer with diagnostic accuracy of 84.8±10.4%.
Among CT findings that were not dependent on reviewers' interpretation, the only one that showed statistical significance in multivariate regression analysis was thrombosis of the false lumen in the proximal ascending aorta as a negative predictor for ascending aorta tear (odds ratio 0.052, confidence interval 0.009-0.308, p=0.001). In univariate analysis, a weak correlation was found between ascending aorta tear and factors such as Marfan syndrome, dissection involvement of all arch branches, and large ascending aortic diameter. No significant predictor for intimal tear was found in the arch (Table 3).
According to our study, well-educated surgeons could be accurate in three fourths of cases. There were room for improvement through experience. Considering the substantial inaccuracy, critical decisions on CT images should be made after thorough reviewing by as many experienced radiologists and surgeons as possible.
The modern MDCT scanners have enabled us to make expeditious and accurate diagnosis of acute aortic dissection and several lesion characteristics.1)2) Recently, many studies have relied on CT to determine the location and characteristics of intimal tears.3)4) However, only a few studies have focused on the validation of the accuracy of CT in predicting intimal tear locations. While studies using old generation scanners concluded that CT was ineffective for detecting intimal tear site,21)22) the accuracy of intimal tear detection varied from 50 to 90% in a small number of recent studies using modern ultrafast or multislice CT. Most of these previous reports validated CT findings with surgical confirmation in less than 20 patients.12)13)14)15) Only Yoshida's study investigated more than 50 patients and reported the sensitivity, specificity, and accuracy for an intimal tear to be 82%, 100%, and 84%, respectively.16) Although their overall accuracy was quite similar to our 80% accuracy, they included patients with intramural hematoma without specifying the locations of the intimal tears. In contrast, we studied only patients with overt dissection that extended from the ascending aorta to the descending aorta. In our results, the sensitivity was higher for ascending aorta tears. However, the specificity was higher for arch tears in both overall average and individual reviewer's judgments.
The presence of large distinctive intimal defects with free flap edges pointing toward the false lumen (Fig. 2A-D) can lead to unanimous or almost unanimous judgments of intimal tear sites. Kapoor et al.23) have named such finding as an "intimomedial rupture" and reported that it could be seen in 8% of patients. In our results, the incidence was much higher. During individual reviewing of the images, investigators pointed out defects (also called discontinuities or gaps) in the intimal flap shadow in an average of 60% of patients. Most of them were specific for surgically found intimal tears except a few patients (Fig. 4).
For intimal defects that were not typical as described by Kapoor et al.,23) thorough review of consecutive cross-sectional images or multiplanar images should help the judgment. Indirect findings such as invagination of mobile flap and abrupt change of the false lumen patency could also be useful (Fig. 2E-G and Fig. 3). When the false lumen in the proximal ascending aorta was thrombosed, the possibility of finding the tear in the ascending aorta was very low (3/18=16.7%). Other indirect findings reported to be significant for predicting intimal tear locations17) were not good predictors in the present study. Although patients with an ascending aorta tear tended to have more frequently pericardial effusion and larger ascending aortic diameter, the correlation was not significant in multiple regression analysis. Judgment depending on only indirect findings without radiological flap defects could lead to inaccurate localization for the tear even by experienced radiologists (Fig. 5).
The limitations of this study are as follows. Since the reviewing surgeons had participated in the surgeries for some patients, they might not be completely blind to surgical findings. However, we believe that such chance is minimal because all the reviewers reviewed the CT images without reviewing the medical records during a 1-2 day period or a long period (at least 1 year) after the operation. Whether surgical findings are reliable enough as reference for validation is questionable. As the tear location was recorded in a systematic record form for acute type A dissection and confirmed by the operating surgeon, we believe that our data have little chance of inaccuracy or having missed description of intimal tears.
In comparison with previous studies based on CT interpretation in consensus of two or more physicians, our study averaged individual reviewers' accuracy. This factor might result in lower accuracy of CT than what can be achieved by validating multipersonal consensus. The heterogeneity of CT scanners and low image qualities due to non-gated or less sliced CT might have resulted in an underestimation of the accuracy of modern MDCT with uniform and proper scanning protocol. However, with all due concerns about such limitations, we aimed to reproduce real clinical practice in which CT images with various quality and protocols reviewed by only a few surgeons without a single radiologist in the emergency preoperative setting.
Although controversy remains with regard to the necessity and additional risk of resection of an arch tear,24)25) we believe that total arch replacement can result in better long-term prognosis. However, it can increase early risk, especially at the hands of less experienced surgeons. Accurate preoperative identification of the intimal tear may affect the clinical practice pattern of a team and thereby the surgical outcome, although it has little impact on the surgical technique. For example, a high possibility of a large arch tear may lead to assigning the patient to a more experienced surgeon who has better outcome of total arch replacement. Total circulatory arrest could be performed at different degrees of hypothermia according to the expected chance of total arch replacement that requires longer circulatory arrest.
In summary, the present study has the following findings:
Our seven reviewers had unanimous consensus or majority (five or six reviewers) consensus for the intimal tear site in 39 of 50 patients, of which 97% of them were correct. Therefore, 80% of real world MDCT images can show findings highly specific for an intimal tear for easy localization.
Surgeons tend to be less accurate in predicting the intimal tear site than radiologists. They also made wrong interpretation for important key findings more frequently (10%) than radiologists (1.5%). However, even trainee surgeons could accurately identify intimal tears on CT images in 75% of patients. We attribute such better-than-expected result to continuous education by experienced surgeons and sharing feedback information acquired from comparison of preoperative prediction and surgical findings. Further improvement can be achieved by continuing such efforts.
When blinded to the interpretation of the others, even radiologists specialized in cardiovascular imaging may not reach correct consensus regarding the intimal tear site. If a critical decision should rely on the location of intimal tears such as in determining the feasibility of endovascular repair in type A dissection, consensus should be reached after thorough reviewing by as many experienced radiologists and surgeons as possible.
Figures and Tables
Fig. 1
The aorta is divided into four segments to categorize the predicted site of intimal tear according to the probability of need for arch replacement. 1; ascending aorta, 2; inferior arch, 3; superior arch, 4; beyond arch/descending thoracic aorta.
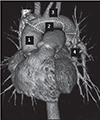
Fig. 2
Distinct intimal flap defect found in the ascending aorta (A), the anterosuperior arch (B), and origin of aberrant right subclavian artery (C and D). Unusual findings in the transverse sections (E and F) turned out to be complete transection and distal invagination of the intimal flap in the sagittal section (G). All reviewers accurately pointed out those findings.
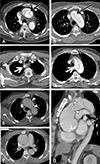
Fig. 3
Tiny flap defect is barely identifiable in the axial section (A and B), but only in the sagittal section (arrow in C and D). While the radiologists were all accurate, some surgeons missed such findings. Thrombosis of false lumen in the proximal ascending aorta is present on C and D.
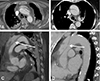
Fig. 4
One of two flap discontinuities shown in the sagittal section is concordant with the real intimal tear (arrows in A and C). The other one (arrowheads in B and C) is caused by folding of the intimal flap, leading to false positive diagnosis by five of the seven reviewers, including one radiologist.
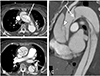
Fig. 5
The cases of retrograde dissection from descending aortic tear for which all radiologists were inaccurate. The point of abrupt change in false lumen patency (A-C, arrow) or point of suspicious contrast leakage (D and E, arrow) turned out to be free from intimal tear.
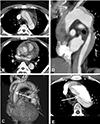
Table 1
Clinical profiles of register

Table 2
Accuracy of predicting intimal tear site with computed tomography findings

Table 3
Factors associated with intimal tear location

Acknowledgment
This work was supported by a grant (02-2009-036) funded by the Seoul National University Bundang Hospital.
References
1. Berger FH, van Lienden KP, Smithuis R, Nicolaou S, van Delden OM. Acute aortic syndrome and blunt traumatic aortic injury: pictorial review of MDCT imaging. Eur J Radiol. 2010; 74:24–39.
2. McMahon MA, Squirrell CA. Multidetector CT of aortic dissection: a pictorial review. Radiographics. 2010; 30:445–460.
3. Khoynezhad A, Walot I, Kruse MJ, Rapae T, Donayre CE, White RA. Distribution of intimomedial tears in patients with type B aortic dissection. J Vasc Surg. 2010; 52:562–568.
4. Quint LE, Platt JF, Sonnad SS, Deeb GM, Williams DM. Aortic intimal tears: detection with spiral computed tomography. J Endovasc Ther. 2003; 10:505–510.
5. Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med. 1999; 340:1546–1552.
6. Ehrlich MP, Rousseau H, Heijmen R, et al. Midterm results after endovascular treatment of acute, complicated type B aortic dissection: The Talent Thoracic Registry. J Thorac Cardiovasc Surg. 2013; 145:159–165.
7. Kato N, Shimono T, Hirano T, Ishida M, Yada I, Takeda K. Transluminal placement of endovascular stent-grafts for the treatment of type A aortic dissection with an entry tear in the descending thoracic aorta. J Vasc Surg. 2001; 34:1023–1028.
8. Lyons O, Clough R, Patel A, Saha P, Carrell T, Taylor P. Endovascular management of Stanford type a dissection or intramural hematoma with a distal primary entry tear. J Endovasc Ther. 2011; 18:591–600.
9. Ye C, Chang G, Li S, et al. Endovascular stent-graft treatment for Stanford type A aortic dissection. Eur J Vasc Endovasc Surg. 2011; 42:787–794.
10. Zimpfer D, Czerny M, Kettenbach J, et al. Treatment of acute type a dissection by percutaneous endovascular stent-graft placement. Ann Thorac Surg. 2006; 82:747–749.
11. Sobocinski J, O'Brien N, Maurel B, et al. Endovascular approaches to acute aortic type A dissection: a CT-based feasibility study. Eur J Vasc Endovasc Surg. 2011; 42:442–447.
12. Jaussaud N, Chitsaz S, Meadows A, et al. Acute type A aortic dissection intimal tears by 64-slice computed tomography: a role for endovascular stent-grafting? J Cardiovasc Surg (Torino). 2013; 54:373–381.
13. Mishra M, Khurana P, Meharwal ZS, Trehan N. A comparative study of imaging techniques in aortic dissection, DeBakey type I: intraoperative live three-dimensional epicardial echocardiography, multiplane transesophageal echocardiography, and multislice computed tomography. Innovations (Phila). 2005; 1:40–47.
14. Sommer T, Fehske W, Holzknecht N, et al. Aortic dissection: a comparative study of diagnosis with spiral CT, multiplanar transesophageal echocardiography, and MR imaging. Radiology. 1996; 199:347–352.
15. Hamada S, Takamiya M, Kimura K, Imakita S, Nakajima N, Naito H. Type A aortic dissection: evaluation with ultrafast CT. Radiology. 1992; 183:155–158.
16. Yoshida S, Akiba H, Tamakawa M, et al. Thoracic involvement of type A aortic dissection and intramural hematoma: diagnostic accuracy--comparison of emergency helical CT and surgical findings. Radiology. 2003; 228:430–435.
17. Takami Y, Tajima K, Kato W, et al. Can we predict the site of entry tear by computed tomography in patients with acute type A aortic dissection? Clin Cardiol. 2012; 35:500–504.
18. Bonser RS, Ranasinghe AM, Loubani M, et al. Evidence, lack of evidence, controversy, and debate in the provision and performance of the surgery of acute type A aortic dissection. J Am Coll Cardiol. 2011; 58:2455–2474.
19. Krähenbühl E, Maksimovic S, Sodeck G, et al. What makes the difference between the natural course of a remaining type B dissection after type A repair and a primary type B aortic dissection? Eur J Cardiothorac Surg. 2012; 41:e110–e115. discussion e115-6
20. Conzelmann LO, Krüger T, Hoffmann I, et al. German registry for acute aortic dissection type A (GERAADA): initial results. Herz. 2011; 36:513–524.
21. Nienaber CA, von Kodolitsch Y, Nicolas V, et al. The diagnosis of thoracic aortic dissection by noninvasive imaging procedures. N Engl J Med. 1993; 328:1–9.
22. Godwin JD, Breiman RS, Speckman JM. Problems and pitfalls in the evaluation of thoracic aortic dissection by computed tomography. J Comput Assist Tomogr. 1982; 6:750–756.
23. Kapoor V, Ferris JV, Fuhrman CR. Intimomedial rupture: a new CT finding to distinguish true from false lumen in aortic dissection. AJR Am J Roentgenol. 2004; 183:109–112.
24. Unosawa S, Hata M, Niino T, Shimura K, Shiono M. Prognosis of patients undergoing emergency surgery for type A acute dissection without exclusion of the intimal tear. J Thorac Cardiovasc Surg. 2013; 146:67–71.
25. Easo J, Weigang E, Hölzl P, et al. Influence of operative strategy for the aortic arch in DeBakey type I aortic dissection: analysis of the German Registry for acute aortic dissection type A. J Thorac Cardiovasc Surg. 2012; 144:617–623.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download