Abstract
Background and Objectives
The diagnosis of ischemic (ICM) and non-ischemic cardiomyopathy (NICM) is conventionally determined by the presence or absence of coronary artery disease (CAD) in the setting of a reduced left systolic function. However the presence of CAD may not always indicate that the actual left ventricular (LV) dysfunction mechanism is ischemia, as other non-ischemic etiologies can be responsible. We investigated patterns of myocardial fibrosis using delayed hyperenhancement (DHE) on cardiac magnetic resonance (CMR) in ICM and NICM.
Subjects and Methods
Patients with systolic heart failure who underwent a CMR were prospectively analyzed. The heart failure diagnosis was based on the modified Framingham criteria and LVEF <35%. LV dysfunction was classified as ICM or NICM based on coronary anatomy.
Results
A total of 101 subjects were analyzed; 34 were classified as ICM and 67 as NICM. The DHE pattern was concordant with the conventional diagnosis in 27 (79.4%) of the patients with ICM and 62 (92.5%) of the patients with NCIM. A discordant NICM DHE pattern was present in 8.8% of patients with ICM, and an ICM pattern was detected 6.0% of the patients with NICM. Furthermore, 11.8% of the patients with ICM and 1.5% of those with NICM demonstrated a mixed pattern.
Determining the etiology of left ventricular (LV) systolic dysfunction is central to appropriately treating patients who present with severe heart failure. The diagnosis of ischemic cardiomyopathy (ICM) or non-ischemic cardiomyopathy (NICM) is conventionally based on the presence or absence of coronary artery disease (CAD) as determined by invasive coronary angiography (CAG) and/or stress testing.1) Patients with significant CAD are diagnosed with as ICM, and the cause of their LV dysfunction is presumed to be impaired coronary perfusion. Patients with LV dysfunction and without CAD are generally diagnosed with NICM. However, the presence or absence of CAD does not necessarily define ICM or NICM and may not completely explain the mechanism leading to LV dysfunction.2) For example, non-ischemic myocardial disease can coexist with CAD or a coronary artery can be recanalized after remote myocardial infarction (MI).
Contrast-enhanced cardiac magnetic resonance (CMR) imaging has emerged as an attractive non-invasive modality to differentiate ICM from NICM because of its ability to directly visualize scarred myocardium related to infarction or fibrosis in patients with various cardiomyopathies.3) Myocardial ischemia in patients with ICM generally begins from the sub-endocardium and extends toward the epicardium. Therefore, subendocardial fibrosis or transmural scar changes in the territory of the diseased coronary artery are a typical ICM pattern. The location of myocardial fibrosis varies in patients with NICM and is mismatched to the blood supply from the coronary arteries. Characterizing specific patterns of delayed hyperenhancement (DHE) using CMR has been widely used to help identify the major causes of LV systolic dysfunction.4)5) CAG alone may not help determine whether impaired LV function is due to MI and hibernation or if the CAD is incidentally associated with concomitant primary myocardial disease. In these situations, evaluating the DHE pattern using CMR (CMR-DHE) may help identify the etiology of the cardiomyopathy.
Accordingly, we investigated DHE patterns in patients with ICM and NICM who were diagnosed based on coronary anatomy and evaluated whether the DHE patterns were consistent with the etiologies established as responsible for impaired myocardial function.
A prospective, international cardiac imaging study of heart failure (IMAGING-HF study) was undertaken in Seoul, Korea and Rochester, NY, USA from 2009 to 2011 to evaluate the diagnostic and management roles of CMR and echocardiography in patients with heart failure. Among patients with various degrees of heart failure who enrolled in the registry, 101 patients with heart failure (mean age, 57±13 years) who met the modified Framingham criteria for the diagnosis of heart failure6)7) and who showed severe myocardial dysfunction with a LV ejection fraction (EF) <35% on echocardiography were selected consecutively. All patients were registered after remaining stable on heart failure medications for at least 1 month. Exclusion criteria were: (1) unstable angina or recently presented MI, (2) hemodynamically unstable patients, (3) presence of contraindications to DHE-CMR, such as renal failure (estimated glomerular filtration rate <30 mL/min), claustrophobia, pacemaker, implantable cardiac defibrillator, or metallic implant. After enrollment, each patient was classified into the ICM or NICM groups based on coronary anatomy using CAG and coronary computed tomography angiography as determined by the patient's primary cardiologist. ICM was defined as follows: (1) patients with a history of MI or revascularization with percutaneous coronary intervention or coronary artery bypass grafting, (2) patients with ≥75% stenosis of the left main or proximal left anterior descending coronary artery, and (3) patients with ≥75% stenosis of two or more epicardial coronary arteries. Patients with none of these criteria were classified into the NICM group. This study protocol was approved by the local institutional review board.
All patients underwent CMR studies using a 1.5 T scanner (Magnetom Avanto, Syngo MR B15 version; Siemens Medical Solutions, Erlangen, Germany) with a 32-channel phased-array receiver coil during repeated breath-holds. After localization, cine images for LV mass and volume were acquired using a steady-state free-precession sequence with 8-10 contiguous short-axis slices to cover the entire LV with a slice thickness of 6 mm and gaps of 4 mm. Delayed gadolinium-enhanced imaging was performed using the phasesensitive inversion recovery technique after injecting 0.15 mmoL/kg Gadovist (gadobutrol; Bayer Healthcare, Berlin, Germany) with continuous short-axis image acquisition of 10-12 slices of 6 mm thickness and a 4 mm gap. Each DHE pattern was evaluated 10 and 15 min after gadolinium administration. Inversion delay time was usually 280-360 ms. Field of view and the image matrix were 35×35 cm and 256×256, respectively.
All measurements were performed at the Samsung Medical Center magnetic resonance imaging (MRI) core laboratory. The images were analyzed to evaluate LV function, mass, and the DHE pattern. LV volume on the MRI images was analyzed using commercial software (Argus ver. 4.02, Siemens, Erlangen, Germany) by a single experienced observer who was blinded to the patient data. End-diastolic and end-systolic frames were defined as frames where the cavity sizes were largest and smallest by retrospective image review, respectively. Endocardial and epicardial borders were traced manually on the selected image frames. Papillary muscles and LV trabeculae were excluded from the endocardium and included in the LV cavity volume.8) LV mass was calculated by multiplying myocardial volume by myocardial density (1.05 g/mL), and the LV mass index was derived from the LV mass divided by body surface area (m2).
DHE images were reviewed by two experienced CMR imagers (training level III) and DHE patterns were diagnosed by agreement of both readers. Three types of DHE patterns were recognized, such as the ICM and NICM patterns, and the mixed pattern. The ICM pattern was defined as involvement of the subendocardium or transmural extension of the infarcted scar located in territories consistent with the perfused area of specific epicardial coronary arteries.9)10) The NICM pattern was defined as patchy or diffuse enhancement or mid-wall involvement without feature of a typical ICM pattern. If the ICM and NICM patterns coexisted in the same myocardium, we classified the pattern as mixed.
All analyses were performed with SPSS software ver. 18.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were analyzed using Student's t-test or the Wilcoxon rank-sum test and expressed as means±standard deviations or medians (interquartile range), where applicable. Categorical data were tested using Fisher's exact test or the chi square test and expressed as numbers in a category and percentages. A two-tailed p<0.05 was considered significant.
Among the 101 patients with severe LV systolic dysfunction, 34 were classified in to the ICM group and 67 were placed in the NICM group based on coronary anatomy. Table 1 summarizes the general characteristics of the patients in the two groups. The ICM group was older (61.4±11.0 vs. 55.5±12.9 years, p=0.026) and included more males and cardiovascular risk factors than those in the NICM group (all p<0.05).
The CMR parameters of the study population are shown in Table 2. Decreased LVEF and a dilated LV cavity indicated severe myocardial systolic dysfunction in both groups. About 79% (n=27) of patients with ICM and 92.5% (n=62) of those with NICM demonstrated a DHE pattern consistent with the conventional diagnosis (Fig. 1). However, a non-concordant DHE pattern was observed in 8.8% of patients with ICM and 6.0% of patients with NICM. Furthermore, 11.8% (n=4) of patients with ICM and 1.5% (n=1) of patients with NICM showed a mixed DHE pattern. A non-concordant DHE pattern was observed in 25% (n=3) of non-post-MI patients with ICM but none of the post-MI patients with ICM. About 14% (n=3) of post-MI patients with ICM and 8.3% (n=1) non-post-MI patients with ICM showed a mixed DHE pattern. Among three patients with ICM who showed a NICM-type DHE pattern, two cases demonstrated focal patchy DHE and one case showed linear DHE involving the mid wall of the ventricular septum. The mixed DHE pattern in patients with ICM was epicardial (two patients) or linear enhancement in the mid septum (two patients) combined with subendocardial or transmural myocardial enhancement. No evidence of significant CAD or subendocardial or transmural enhancement, which is a pattern consistent with ICM, was demonstrated in four patients with NICM. Representative CMR images of DHE patterns discordant from the conventional diagnosis are shown in Figs 2 and 3. All four patients conventionally diagnosed with NICM who showed typical subendocardial DHE did not have any clinical history of CAD. All patients with ICM with a NICM-type pattern of DHE had a history of CAD with completely revascularized coronary stenoses, but did not have any ICM pattern DHE in infarcted myocardium that could explain the residual severe LV systolic dysfunction.
Our findings indicate that the etiology of severe LV systolic dysfunction can be misclassified if it is solely based on coronary artery anatomy, as some patients diagnosed with ICM or NICM by conventional methods according to the presence or absence of coronary artery stenotic disease demonstrate discordant DHE patterns. Some patients with ICM and NICM also demonstrate non-specific mixed DHE patterns.
CMR-DHE imaging allows visualization of fibrosis; thus, macro-scars, such as MIs, are detectable in most patients with acute MI.11) Although the volume of a MI scar shrinks with time, it remains present in the myocardium as and may extend to the peri-infarct zone. Ischemic myocardium without infarction is viable (hibernating myocardium), which indicates probable recovery of contractility after revascularization.12) These associations have also been found in other CMR studies; most hypokinetic myocardial segments without scars are "viable" after revascularization.13) In our study, a few patients continued to have severe LV systolic dysfunction even after undergoing complete revascularization but had no myocardial fibrosis. A similar group of patients with "ICM," minimal scaring, and persistent myocardial dysfunction after revascularization have been reported previously,11)13) indicating that recovery of myocardial function after revascularization is not always complete, even in fibrosis-free myocardium. There are several possible explanations for this phenomenon. First, CMR may have limited ability to evaluate diffuse subendocardial scars that can lead to myocardial dysfunction. Furthermore, as the LV cavity is also "bright" on DHE images, the bright dead tissue detected by DHE may remain unidentified, particularly in cases of small or shallow subendocardial infarctions.14) Even in these cases, multi-vessel disease can cause diffuse subendocardial infarction, which could not be observed in our study. Another explanation is co-existence of ischemic myocardial disease related to a non-ischemic etiology. If the myocardial DHE pattern indicates non-ischemic cardiomyopathy in patients with CAD, as in some of our patients, a co-existing myocardial process should be strongly suspected. A third possible explanation is that functional recovery of hibernated myocardium is delayed so LV systolic dysfunction persists, as the time course of functional recovery of hibernating myocardium after revascularization may vary from days to months.15)
The diagnosis of NICM using criteria of LV dysfunction and "normal coronary arteries" based on coronary angiography may result in misdiagnosing ICM if a coronary artery stenosis was revascularized or was related to vasospasm. Some studies have reported that the presence of typical post-infarct DHE in patients with NICM is a sign of an unrecognized previous MI and subsequent recanalization.16)17) McCrohon et al.2) reported that use of CAG to determine the presence of LV dysfunction caused by CAD leads to an incorrect diagnosis of dilated cardiomyopathy in 13% of patients, possibly because of coronary recanalization after MI. Therefore, some researchers have proposed that there may be a considerable number of patients with ICM misdiagnosed by CAG as having NICM.16)18)
Another study19) suggested that simply distinguishing between cardiomyopathies based on the presence or absence of CAD has limitations. They showed that the prognosis of some patients diagnosed with ICM is similar to those with NICM, particularly in cases of a small territory CAD. The combination of coronary angiography with CMR (DHE imaging) can help establish a treatment plan in such patients with heart failure. Even if obstructive CAD is present, other causes of LV dysfunction in patients with functionally normal coronary perfusion should be investigated rather than assuming a diagnosis of ICM. Furthermore, the possibility of the coexistence of mixed cardiomyopathy with NICM should be considered, as this may contribute independently to LV systolic dysfunction.3)
DHE patterns determined by CMR can provide additional information to determine the exact etiology of cardiomyopathies by allowing the assessment of myopathic processes that reflect the histopathology of ischemic and non-ischemic heart disease.202122) As CAG may not help determine the exact etiology in patients with severe LV dysfunction, CMR with DHE should be considered before coronary angiography in this patient population.
The number of patients conventionally diagnosed with ICM was relatively small; however, all patients were enrolled consecutively, and our study focused on discrete manifestations of DHE. Thus, a comparative group analysis was not central to address our aims. The prevalence of DHE in patients with NICM was higher than reported previously, which may have been due to the strict enrollment criteria which included only patients with LVEF <35% and excluded those with mild to moderate LV systolic dysfunction to clarify myocardial changes related to underlying disease. Our results suggest that a mismatched DHE pattern can indicate the existence of different or mixed causes of LV dysfunction in patients with heart failure; however, our limited sample size prevents us from generalizing our findings. Therefore, a large-scale study involving more patients with LV systolic dysfunction is warranted.
A subset of patients conventionally diagnosed with ICM or NICM based on coronary anatomy may have a mixed or a different etiology based on a DHE-CMR examination. This may explain persistent LV dysfunction after coronary revascularization. DHE-CMR should be considered an important diagnostic modality to evaluate severe LV dysfunction prior to coronary angiography.
Figures and Tables
Fig. 1
Delayed hyperenhancement pattern on CMR in patients with cardiomyopathy and severe left ventricular systolic dysfunction. CMR: cardiac magnetic resonance, ICM: ischemic cardiomyopathy, NICM: nonischemic cardiomyopathy.

Fig. 2
Representative cases of ICM patients with NICM or mixed patterns of delayed hyperenhancement on CMR. (A) The basal mid-septum is enhanced linearly (pattern of non-ischemic cardiomyopathy) on this 10-min delayed short axis image (arrowheads). (B) Basal myocardium shows linear enhancement of the mid-septum (arrowheads) and transmural enhancement (black arrows) (mixed pattern) on this 10-min delayed short axis image. ICM: ischemic cardiomyopathy, NICM: non-ischemic cardiomyopathy, CMR: cardiac magnetic resonance.
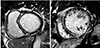
Fig. 3
Representative cases of NICM patients ICM or mixed patterns of delayed hyperenhancement on CMR. (A) Lateral segment of the mid myocardium demonstrates subendocardial delayed hyperenhancement (pattern of ischemic cardiomyopathy, black arrows) on this 10-min delayed short axis image. (B) Multiple focal patchy hyperenhancement in the septum (arrowheads) and transmural hyperenhancement (black arrows) of the inferolateral segment of the basal myocardium are evident in this 10-min delayed short axis image. NICM: non-ischemic cardiomyopathy, ICM: ischemic cardiomyopathy, CMR: cardiac magnetic resonance.

Table 1
Clinical characteristics of the study population

Table 2
Cardiac magnetic resonance data

Data are expressed as mean±standard deviation. ICM: ischemic, NICM: non-ischemic cardiomyopathy, LA: left atrium, LV: left ventricle, ESV: end systolic volume, EDV: end diastolic volume, LVEF: left ventricle ejection fraction, LAVI: left atrium volume index, ESVI: end systolic volume index, EDVI: end diastolic volume index, LVMI: left ventricle mass index
References
1. Nieminen MS, Böhm M, Cowie MR, et al. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J. 2005; 26:384–416.
2. McCrohon JA, Moon JC, Prasad SK, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003; 108:54–59.
3. Senthilkumar A, Majmudar MD, Shenoy C, Kim HW, Kim RJ. Identifying the etiology: a systematic approach using delayed-enhancement cardiovascular magnetic resonance. Heart Fail Clin. 2009; 5:349–367, vi.
4. Wu E, Judd RM, Vargas JD, Klocke FJ, Bonow RO, Kim RJ. Visualisation of presence, location, and transmural extent of healed Q-wave and non-Q-wave myocardial infarction. Lancet. 2001; 357:21–28.
5. Yoon YE, Hong YJ, Kim HK, et al. 2014 Korean Guidelines for Appropriate Utilization of Cardiovascular Magnetic Resonance Imaging: a joint report of the Korean Society of Cardiology and the Korean Society of Radiology. Korean Circ J. 2014; 44:359–385.
6. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001; 285:2486–2497.
7. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002; 106:3143–3421.
8. Janik M, Cham MD, Ross MI, et al. Effects of papillary muscles and trabeculae on left ventricular quantification: increased impact of methodological variability in patients with left ventricular hypertrophy. J Hypertens. 2008; 26:1677–1685.
9. Cummings KW, Bhalla S, Javidan-Nejad C, Bierhals AJ, Gutierrez FR, Woodard PK. A pattern-based approach to assessment of delayed enhancement in nonischemic cardiomyopathy at MR imaging. Radiographics. 2009; 29:89–103.
10. Jackson E, Bellenger N, Seddon M, Harden S, Peebles C. Ischaemic and non-ischaemic cardiomyopathies--cardiac MRI appearances with delayed enhancement. Clin Radiol. 2007; 62:395–403.
11. Kim HW, Farzaneh-Far A, Kim RJ. Cardiovascular magnetic resonance in patients with myocardial infarction: current and emerging applications. J Am Coll Cardiol. 2009; 55:1–16.
12. Braunwald E, Rutherford JD. Reversible ischemic left ventricular dysfunction: evidence for the "hibernating myocardium". J Am Coll Cardiol. 1986; 8:1467–1470.
13. Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000; 343:1445–1453.
14. Wagner A, Mahrholdt H, Thomson L, et al. Effects of time, dose, and inversion time for acute myocardial infarct size measurements based on magnetic resonance imaging-delayed contrast enhancement. J Am Coll Cardiol. 2006; 47:2027–2033.
15. Bax JJ, Visser FC, Poldermans D, et al. Time course of functional recovery of stunned and hibernating segments after surgical revascularization. Circulation. 2001; 104:12 Suppl 1. I314–I318.
16. Soriano CJ, Ridocci F, Estornell J, Jimenez J, Martinez V, De Velasco JA. Noninvasive diagnosis of coronary artery disease in patients with heart failure and systolic dysfunction of uncertain etiology, using late gadolinium-enhanced cardiovascular magnetic resonance. J Am Coll Cardiol. 2005; 45:743–748.
17. Calore C, Cacciavillani L, Boffa GM, et al. Contrast-enhanced cardiovascular magnetic resonance in primary and ischemic dilated cardiomyopathy. J Cardiovasc Med (Hagerstown). 2007; 8:821–829.
18. Soriano CJ, Ridocci F, Estornell J, et al. Late gadolinium-enhanced cardiovascular magnetic resonance identifies patients with standardized definition of ischemic cardiomyopathy: a single centre experience. Int J Cardiol. 2007; 116:167–173.
19. Felker GM, Shaw LK, O'Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002; 39:210–218.
20. Mahrholdt H, Goedecke C, Wagner A, et al. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004; 109:1250–1258.
21. Moon JC, Reed E, Sheppard MN, et al. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004; 43:2260–2264.
22. Kim RJ, Fieno DS, Parrish TB, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999; 100:1992–2002.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download