Abstract
Water extract of guibi-tang (GB), a traditional Chinese, Japanese, and Korean herbal medicine, is used to treat memory impairment, insomnia, and peptic ulcers. The aim of this study was to investigate the protective effects of GB on pulmonary inflammation induced by cigarette smoke (CS) and lipopolysaccharide (LPS). C57BL/6 mice were used to develop a pulmonary inflammation model by exposing them to CS for 1 h per day for 7 days. LPS was intranasally administered to mice under mild anesthesia on day 5. GB was administered 1 h before CS exposure at doses of 50 or 100 mg/kg for 7 days. Our results showed that GB suppressed the CS and LPS induced elevation in inflammatory cell counts in the bronchoalveolar lavage fluid (BALF), with significant reductions in protein, tumor necrosis factor (TNF)-α, and interleukin (IL)-6 levels. Histological studies revealed that GB decreased the inflammatory cell infiltration into lung tissue caused by CS- and LPS-exposure. GB also significantly decreased the CS and LPS-induced expression of inducible nitric oxide synthase (iNOS) in the lung tissue. Taken together, GB effectively attenuated airway inflammation caused by CS and LPS. These results indicate that GB is a potential therapeutic herbal formula for pulmonary inflammatory disease.
The incidence of airway inflammation has been rising gradually in recent years owing to increases in the atmospheric levels of various toxic materials including pollutants, chemicals, cigarette smoke, and allergens. In particular, chronic obstructive pulmonary disease (COPD), a respiratory disorder characterized by airway inflammation, has become the third leading cause of mortality in the USA [1]. Cigarette smoke (CS) is considered to be a primary risk factor for COPD, leading to airflow limitation and emphysema by inducing airway inflammation [23]. Because CS contains chemicals such as oxidants, aldehydes, nicotine, and carbon monoxide, the lung tissues of smokers have elevated levels of inflammatory mediators, such as chemokines and proinflammatory cytokines, which are released by the lung epithelial cells [45]. These mediators eventually accelerate airway inflammation via increased recruitment of inflammatory cells, particularly neutrophils and macrophages [6].
Neutrophils contribute to the loss of lung function and destruction of normal alveolar structure in COPD patients by releasing elevated amounts of inflammatory molecules [7]. The oxidant-generating systems of neutrophils produce reactive nitrogen species such as nitric oxide (NO) [8]. Inducible nitric oxide synthase (iNOS) regulated by NO and excessive production of NO are known to be associated with airway inflammation such as in COPD [91011]. A recent study showed that CS-exposed animals exhibited airway inflammation with an elevated expression of iNOS. [12]. Therefore, down-regulation of iNOS is considered an important strategy in treating CS-induced airway inflammation.
Water extract of guibi-tang (GB), a traditional herbal formula, is a mixture of 12 herbs and is known as Qui-Pi-tang in Chinese and Kihi-To in Japanese. [1314]. GB has been traditionally used to treat amnesia, fatigue, insomnia, anemia, and palpitations [15]. Studies have shown that GB has various pharmacological activities, including immunoregulatory, antioxidant, and gastroprotective effects. GB inhibits ischemic retinal angiogenesis by suppressing VEGF and PAI-1 expression and exhibits apoptotic activity by regulating mitogenactivated protein kinases (MAPKs) [1316]. However, thus far, no studies have reported the protective effects of GB against pulmonary inflammation induced by CS and lipopolysaccharide (LPS).
Therefore, we investigated the protective effects of GB on CS- and LPS-induced pulmonary inflammation in a mouse model. We evaluated the expression of several inflammatory mediators in CS- and LPS-exposed mice to explore the mechanisms of action of GB in pulmonary tissue.
The 12 raw herbs, Angelicae Gigantis Radix, Longan Arillus, Zizyphi Semen, Polygalae Radix, Ginseng Radix, Astragali Radix, Atractylodis Rhizoma Alba, Poria sclertum cum Pini Radix, Aucklandiae Radix, Glycyrrhizae Radix et Rhizoma, Zingiberis Rhizoma Recens, and Zizyphi Fructus were purchased from Omniherb (Yeongcheon, Korea) and from HMAX (Jecheon, Korea) in February 2008. Each raw material was verified by Professor Je-Hyun Lee, Oriental Medicine, Dongguk University (Gyeongju, Korea) and by Professor Young-Bae Seo, Oriental Medicine, Daejeon University (Daejeon, Korea). Voucher specimens (2008-KE22-1-KE22-12) have been deposited at the K-herb Research Center, Korea Institute of Oriental Medicine (KIOM, Daejeon, Korea).
GB was prepared and stored at KIOM. Briefly, Angelicae Gigantis Radix (872 g), Longan Arillus (872 g), Zizyphi Semen (872 g), Polygalae Radix (872 g), Ginseng Radix (872 g), Astragali Radix (872 g), Atractylodis Rhizoma Alba (872 g), Poria sclertum cum Pini Radix (872 g), Aucklandiae Radix (436 g), Glycyrrhizae Radix et Rhizoma (262 g), Zingiberis Rhizoma Recens (1,453 g), and Zizyphi Fructus (872 g) were mixed and extracted in a 10-fold mass (100 L) of water at 100℃ for 2 h using the reflux method. The extracted water solution was freeze-dried to yield a powder (mass of extract: 2.43 kg; yield: 24.3%).
Liquiritin (99.6%) and glycyrrhizin (99.0%) were purchased from Wako Chemicals (Osaka, Japan). Nodakenin (98.0%) was purchased from NPC Bio Technology (Yeongi, Korea). The standard solution for reference was dissolved in methanol at a concentration of 1,000 µg/mL and stored at 4℃. The HPLC system used for the quantitative analysis of the GB sample was a Shimadzu Prominence LC-20A series instrument (LC-20A, SHIMAZU, Kyoto, Japan) coupled with a photodiode array detector. Gemini C18 (250 mm×4.6 mm; 5 µm, Phenomenex, Torrance, CA) was used for the separation of analytes and maintained at 40℃. The mobile phase system consisted of distilled water and acetonitrile, both containing 1% (v/v) acetic acid. The gradient elution program for the mobile phases was as follows: 10–70% B (0–30 min), 70–100% B (30–35 min), 100% B (for 35–40 min), 100–10% B (40–45 min), and 10% B (45–60 min). The flow rate was 1 mL/min, and the injection volume was 10 µL. For evaluation, 400 mg of freeze-dried GB extract was dissolved in 20 mL distilled water, and the solution was filtered through a 0.2-µm membrane filter (PALL Life Sciences, Ann Arbor, MI) before injecting it to the HPLC system.
Specific pathogen-free C57BL/6N mice were obtained from Samtako Co. (Osan, Korea). The animals were 6–8 weeks old and weighed 20–25 g. The mice were housed under standard conditions of temperature (22±2℃) and humidity (55±5%) and subjected to a 12-h light/dark cycle. The Institutional Animal Care and Use Committee of Chonnam National University approved the protocols for our animal study (CNU IACUC-YB-R-2016-18), and the animals were cared for in accordance with the Guidelines for Animal Experiments prescribed by Chonnam National University. The mice were divided into the following five groups: NC (non-treated mice), CS (mice exposed to CS and LPS), ROF (mice exposed to CS and LPS that received roflumilast, SML1099, Sigma-Aldrich Korea, Seoul, Korea, 10 mg/kg orally), and GB50 and GB100 (mice exposed to CS and LPS that received GB 50 and 100 mg/kg, respectively orally). The doses of GB were determined based on a previous report [13]. Roflumilast is a PDE4 inhibitor and used for COPD treatment. Roflumilast not only has bronchodilatory effects but also has protective effects against airway inflammation [1718]. Therefore, roflumilast was used as the positive control drug. For CS exposure, we used a 3R4F research cigarette, containing 9.4 mg of tar and 0.76 mg of nicotine per cigarette, and a cigarette smoke generator (Daehan Biolink, Eumseong, Republic of Korea). The mice were exposed to the cigarette fumes in a chamber (50 cm×30 cm×30 cm) for 1 h (one puff/min; 35 mL puff volume over 2 seconds; 8 cigarettes per day for 7 days). LPS (5 µg, L2630, Sigma-Aldrich Korea) was administered intranasally under mild anesthesia on day 5. Roflumilast and GB were administered by oral gavage before CS exposure on all 7 days of the experimental period.
BALF was collected by tracheostomy of sacrificed mice 48 h after the final intranasal LPS administration. PBS (ice-cold, 0.7 mL) was infused into the lung by tracheal cannulation, and this step was repeated once (total volume 1.4 mL). To determine differential cell count, 100 µL of BALF was centrifuged on slides using a cytospin instrument (A-cyto-12, Hanil Science Industrial, Seoul, Korea). Once dried, the cells were stained with Diff-Quik® (Sysmex Corporation, Kobe, Japan) reagent. The BALF supernatant was stored at −70℃.
Pro-inflammatory cytokine levels in BALF were measured using ELISA kits (TNF-α; DY410, IL-6; DY406, R&D System, Minneapolis, MN) according to the manufacturer's protocols. Following reaction, the plates were incubated for 10 min in the dark, and absorbance was measured at 450 nm using a microplate reader (iMark™, Bio-Rad, Hercules, CA). The amount of pro-inflammatory cytokines was calculated by measuring absorbance using a standard curve.
Protein concentrations in homogenized (10% w/v) lung tissues were determined by using the Bradford method (#5000205, Bio-Rad). The proteins present in the tissue samples were resolved via 10% SDS-polyacrylamide gel electrophoresis, following which the electrophoresed proteins were transferred to polyvinyl difluoride (PVDF, IPVH00010, Merck, Darmstadt, Germany) membranes and incubated with a blocking solution (5% skim milk, 232100, BD Bioscience, San Jose, CA, USA) for 1 h. Appropriate primary antibodies were used to probe the membranes, and they were incubated overnight at 4℃. The primary antibodies and dilutions used were as follows: anti-β-actin (1:2,000 dilution; #4967, Cell Signaling, Danver, MA) and anti-iNOS (1:1,000 dilution; ab15323, abcam, Cambridge, UK). The membranes were washed three times using Tris-buffered saline containing Tween 20 (TBST) and incubated with 1:10,000 diluted horseradish peroxidase (HRP)-conjugated secondary antibody (Jackson Immuno Research, West Grove, PA) for 1 h at room temperature. The membranes were washed three times with TBST, and then developed using an enhanced chemiluminescence (ECL) kit (Thermo Fisher Scientific, Waltham, MA). Protein expression was calculated using densitometric band values obtained from the analysis of developed membranes in a ChemiDoc machine (ChemiDoc XRS+, Bio-Rad).
Fixed lung tissue samples were embedded in paraffin and sectioned into 4-µm-thick slices. The sections were stained with hematoxylin and eosin (H&E solution, Sigma-Aldrich) to evaluate inflammation. For the immunohistochemical assay, the paraffin-embedded lung tissues were deparaffinized, dehydrated, and washed in PBS containing 0.05% Tween 20 (PBS-T). The slides were incubated with goat serum for 20 min at room temperature to block nonspecific staining. They were then incubated for 2 h at room temperature with primary mouse antirabbit iNOS antibody (diluted 1:100, Abcam). Next, they were washed three times using PBS-T, incubated for 1 h at room temperature with a biotinylated secondary antibody, and again incubated with an avidin-biotin-peroxidase complex (Vector Laboratories, Burlingame, CA) for 1 h at room temperature. The slides were washed, incubated with diaminobenzidine (DAB, Abcam) for an additional 5 min, and examined using a microscope.
Quantitative analysis of three marker compounds in the GB sample was conducted using our optimized HPLC method. The three marker compounds, liquiritin, nodakenin, and glycyrrhizin, were eluted at 14.272, 15.205, and 30.708 min, respectively (Figure 1), and the amounts of these components in the GB sample were 1.05, 3.38, and 0.83 mg/g, respectively. These results indicate that the GB sample used in this experiment is the same as the herbal formula available on the market.
The number of inflammatory cells was significantly higher in the CS group mice than in the NC group mice. In particular, mice from the CS group had higher neutrophil counts than mice from the NC group. Although the ROF group showed decreased inflammatory cell count in the BALF, the decrease was not significant compared to that observed in the other groups. However, mice from the GB-treated groups showed a decline in inflammatory cell counts in the BALF compared to the counts in mice from the CS group. Mice from the GB100 group, in particular, showed significantly lower neutrophil counts than mice from the CS group (Figure 2).
The CS group mice showed significantly higher production of TNF-α than mice from the NC group (Figure 3A). The ROF group showed a marked decrease in the production of TNF-α compared to that of the CS group. In addition, mice from the GB-treated groups showed significantly lower TNF-α expression than mice from the CS group. Consistent with these results, IL-6 production was also significantly higher in the CS group mice than in the NC group mice (Figure 3B). By contrast, mice from the GB-treated groups showed significantly lower IL-6 levels in BALF than mice from the CS group.
The results of the western blot analysis showed that the expression of iNOS was significantly higher in mice from the CS group than in mice from the NC group. The ROF group showed a significant reduction in iNOS expression compared to that in the CS group. In addition, mice from the GB-treated groups expressed significantly lower levels of iNOS than mice from the CS group (Figure 4A). Consistent with these results, iNOS expression in lung tissues was significantly higher in the CS group mice than in the lung tissues of NC group mice. By contrast, mice from the ROF and GB-treated groups had significantly lower levels of iNOS in their lung tissues than mice from the CS group (Figure 4B).
The CS group mice showed higher inflammatory cell accumulation in the lung tissue than mice from the NC group. The ROF group showed a marked decrease in inflammatory cell infiltration into lung tissue compared to that in the CS group. In addition, mice from the GB-treated groups showed a decrease in inflammatory cell accumulation in the peribronchial and alveolar lesions compared to that observed in mice from the CS group. This suppression was most evident in the GB100 group (Figure 5).
The incidence of COPD has been on the rise in recent years; COPD is considered a life-threatening disease due to its high morbidity and mortality [1920]. In this study, we investigated the protective effect of the herbal medicine GB against airway inflammation caused by CS and LPS. GB decreased inflammatory cell count and proinflammatory cytokine levels in the BALF of mice exposed to CS and LPS. It also effectively suppressed CS- and LPS-induced iNOS expression in the lung tissue, which was accompanied by a decrease in inflammatory cell accumulation.
CS is regarded as a critical factor in the pathogenesis of COPD, which is characterized by inflammatory responses, airway obstruction, and alteration of normal alveolar structure. [21]. Chronic exposure to CS triggers the recruitment of inflammatory cells such as neutrophils and macrophages, as CS consists of various toxic materials [22]. The infiltration of inflammatory cells into the lung tissue of COPD patients or smokers was higher than that in healthy humans [23]. Of the recruited inflammatory cells, neutrophils are involved in the development of COPD [24]. Neutrophils act as front-line defensive cells of the immune system, but excessive accumulation of neutrophils induces airway inflammation and structural alteration through the release of chemotactic mediators such as ROS, inflammatory cytokines, and tissue-damaging enzymes [2425]. Therefore, the suppression of neutrophil count is considered an important approach in controlling airway inflammation induced by CS. In this study, GB treatment effectively suppressed these elevated inflammatory cell counts in the BALF of mice exposed to CS and LPS. This was accompanied by a reduction in the accumulation of inflammatory cells in peribronchial and alveolar lesions of mice exposed to CS and LPS. These results demonstrate that GB effectively inhibited inflammatory cell accumulation induced by CS and LPS.
Proinflammatory cytokines such as TNF-α and IL-6 play a vital role in the regulation of lung inflammation induced by CS [5]. TNF-α is an important mediator of airway inflammation in COPD owing to its induction of adhesion molecules via activation of NF-κB and elevation of ROS production [2627]. In addition, IL-6 production is involved in airway inflammation and decline of lung function in COPD [28]. In COPD patients, IL-6 levels are significantly higher than the levels in healthy humans. This upregulation of IL-6 is associated with increased mortality in COPD patients [29]. In an experimental animal model, IL-6 induced not only airway inflammation but also emphysema-like airspace enlargement [30]. Thus, the extent to which TNF-α and IL-6 are downregulated serves as an important indicator of therapeutic efficacy against airway inflammation induced by CS exposure. In this study, GB treatment significantly decreased the production of TNF-α and IL-6 in BALF compared to that in CS- and LPS-exposed mice. Therefore, these results indicated that GB effectively suppressed the production of proinflammatory cytokines induced by CS and LPS exposure.
The expression of iNOS is modulated by various factors including cytokines, chemokines, and exogenous toxic materials; iNOS overexpression resulted in excessive NO production, which consequently elevated oxidative stress via NOS production and aggravated inflammatory response via activation of inflammation-related signaling [31]. iNOS should be studied in clinical trials of possible therapies because iNOS expression is closely associated with the development of COPD. It is mainly overexpressed in small airways and peripheral lung tissue of COPD patients and causes protein degradation and skeletal muscle apoptosis. [3233]. In vivo experiments with iNOS-deficient mice demonstrated inhibition of pulmonary hypertension, ventricular pressure, and vascular remodeling induced by CS exposure [34]. In this study, GB treatment significantly reduced iNOS expression induced by CS and LPS exposure. This suggests that GB effectively decreases airway inflammation resulting from CS and LPS exposure by suppressing iNOS expression.
The preventive effects of GB observed in this study were closely associated with its active components, liquiritin, nodakenin, and glycyrrhizin, which were reported to have anti-inflammatory properties in various experiments. Liquiritin effectively suppressed inflammatory responses in ultraviolet B radiation-exposed skin and decreased the levels of inflammatory mediators in cigarette smoke extract-stimulated lung epithelial cells [3536]. Nodakenin and glycyrrhizin exhibited suppressive effects on airway inflammation in a murine model of allergic asthma [3738]. Based on the properties of three components, we hypothesized that GB effectively inhibits airway inflammation in allergic asthma. Therefore, we will investigate the therapeutic effect of GB on allergic asthma in further study. In addition, we will explore the antioxidant effects of GB because its three components have antioxidant effects [3639].
Based on our results, GB effectively prevented airway inflammation induced by CS and LPS exposure. However, it is difficult to conclude that GB is effective in treating COPD because our experiment is performed with the aim of evaluating the preventive effect on airway inflammation induced by CS and LPS. Thus, additional experiment of therapeutic effect using chronic CS and LPS exposure model should be performed.
Figures and Tables
Figure 2
GB decreased inflammatory cell counts in the BALF of CS- and LPS-exposed mice. NC: non-treated mice; CS: CS- and LPS-exposed mice; ROF: CS- and LPS-exposed mice administered roflumilast (10 mg/kg, per os); GB50 and GB100: CS- and LPS-exposed mice administered GB (50 and 100 mg/kg, per os, respectively). The values are given as means±SD. ##P<0.01 vs NC; *P<0.05 vs CS.

Figure 3
GB reduced the production of inflammatory cytokines caused by exposure to CS and LPS. (A) TNF-α level, (B) IL-6 level. NC: non-treated mice; CS: CS- and LPS-exposed mice; ROF: CS- and LPS-exposed mice administered roflumilast (10 mg/kg, per os); GB50 and GB100: CS- and LPS-exposed mice administered GB (50 and 100 mg/kg, per os, respectively). The values are given as means ± SD. ##P<0.01 vs NC; *P<0.05 vs CS; **P<0.01 vs CS.
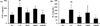
Figure 4
GB suppressed iNOS expression in the lung tissue from CS- and LPS-exposed mice. (A) iNOS expression in the membrane, (B) iNOS expression in the lung tissue. To determine protein expression, we used five samples per group and the experiments were repeated twice. NC: non-treated mice; CS: CS- and LPS-exposed mice; ROF: CS- and LPS-exposed mice administered roflumilast (10 mg/kg, per os); GB50 and GB100: CS- and LPS-exposed mice administered GB (50 and 100 mg/kg, per os, respectively). The values are given as means±SD. ##P<0.01 vs NC; **P<0.01 vs CS.
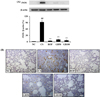
Figure 5
GB attenuated inflammatory cell infiltration. NC: non-treated mice; CS: CS- and LPS-exposed mice; ROF: CS- and LPS-exposed mice administered roflumilast (10 mg/kg, per os); GB50 and GB100: CS- and LPS-exposed mice administered GB (50 and 100 mg/kg, per os, respectively). The values are given as means±SD. ##P<0.01 vs NC; *P<0.05 vs CS; **P<0.01 vs CS.
References
2. Abdul Roda M, Sadik M, Gaggar A, Hardison MT, Jablonsky MJ, Braber S, Blalock JE, Redegeld FA, Folkerts G, Jackson PL. Targeting prolyl endopeptidase with valproic acid as a potential modulator of neutrophilic inflammation. PLoS One. 2014; 9(5):e97594.

3. Boskabady MH, Gholami Mhtaj L. Effect of the Zataria multiflora on systemic inflammation of experimental animals model of COPD. Biomed Res Int. 2014; 2014:802189.
4. Liu H, Ren J, Chen H, Huang Y, Li H, Zhang Z, Wang J. Resveratrol protects against cigarette smoke-induced oxidative damage and pulmonary inflammation. J Biochem Mol Toxicol. 2014; 28(10):465–471.

5. Liu MH, Lin AH, Lee HF, Ko HK, Lee TS, Kou YR. Paeonol attenuates cigarette smoke-induced lung inflammation by inhibiting ROS-sensitive inflammatory signaling. Mediators Inflamm. 2014; 2014:651890.

6. Hsiao HM, Sapinoro RE, Thatcher TH, Croasdell A, Levy EP, Fulton RA, Olsen KC, Pollock SJ, Serhan CN, Phipps RP, Sime PJ. A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PLoS One. 2013; 8(3):e58258.

7. Nikota JK, Shen P, Morissette MC, Fernandes K, Roos A, Chu DK, Barra NG, Iwakura Y, Kolbeck R, Humbles AA, Stampfli MR. Cigarette smoke primes the pulmonary environment to IL-1α/CXCR-2-dependent nontypeable Haemophilus influenzae-exacerbated neutrophilia in mice. J Immunol. 2014; 193(6):3134–3145.

8. Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011; 17(3-4):293–307.

9. Berry M, Hargadon B, Morgan A, Shelley M, Richter J, Shaw D, Green RH, Brightling C, Wardlaw AJ, Pavord ID. Alveolar nitric oxide in adults with asthma: evidence of distal lung inflammation in refractory asthma. Eur Respir J. 2005; 25(6):986–991.

10. Bhattacharjee A, Prasad SK, Pal S, Maji B, Banerjee A, Das D, Bose A, Chatterjee N, Mukherjee S. Possible involvement of iNOS and TNF-α in nutritional intervention against nicotine-induced pancreatic islet cell damage. Biomed Pharmacother. 2016; 84:1727–1738.

11. Lee H, Park JR, Kim EJ, Kim WJ, Hong SH, Park SM, Yang SR. Cigarette smoke-mediated oxidative stress induces apoptosis via the MAPKs/STAT1 pathway in mouse lung fibroblasts. Toxicol Lett. 2016; 240(1):140–148.

12. Gupta I, Ganguly S, Rozanas CR, Stuehr DJ, Panda K. Ascorbate attenuates pulmonary emphysema by inhibiting tobacco smoke and Rtp801-triggered lung protein modification and proteolysis. Proc Natl Acad Sci USA. 2016; 113(29):E4208–E4217.

13. Lee YM, Lee YR, Kim CS, Jo K, Sohn E, Kim JS, Kim J. Effect of Guibi-Tang, a Traditional Herbal Formula, on Retinal Neovascularization in a Mouse Model of Proliferative Retinopathy. Int J Mol Sci. 2015; 16(12):29900–29910.

14. Lee MY, Seo CS, Kim JY, Shin HK. Genotoxicity evaluation of Guibi-Tang extract using an in vitro bacterial reverse mutation assay, chromosome aberration assay, and in vivo micronucleus test. BMC Complement Altern Med. 2014; 14:215.

15. Oh MS, Huh Y, Bae H, Ahn DK, Park SK. The multi-herbal formula Guibi-tang enhances memory and increases cell proliferation in the rat hippocampus. Neurosci Lett. 2005; 379(3):205–208.

16. Yim NH, Kim A, Liang C, Cho WK, Ma JY. Guibitang, a traditional herbal medicine, induces apoptotic death in A431 cells by regulating the activities of mitogen-activated protein kinases. BMC Complement Altern Med. 2014; 14:344.

17. Abbott-Banner KH, Page CP. Dual PDE3/4 and PDE4 inhibitors: novel treatments for COPD and other inflammatory airway diseases. Basic Clin Pharmacol Toxicol. 2014; 114(5):365–376.

18. Kubo S, Kobayashi M, Iwata M, Miyata K, Takahashi K, Shimizu Y. Anti-neutrophilic inflammatory activity of ASP3258, a novel phosphodiesterase type 4 inhibitor. Int Immunopharmacol. 2012; 12(1):59–63.

19. Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev. 2007; 87(3):1047–1082.

20. John-Schuster G, Günter S, Hager K, Conlon TM, Eickelberg O, Yildirim AÖ. Inflammaging increases susceptibility to cigarette smoke-induced COPD. Oncotarget. 2016; 7(21):30068–30083.

21. Leberl M, Kratzer A, Taraseviciene-Stewart L. Tobacco smoke induced COPD/emphysema in the animal model-are we all on the same page? Front Physiol. 2013; 4:91.

22. Li Y, Yu G, Yuan S, Tan C, Lian P, Fu L, Hou Q, Xu B, Wang H. Cigarette Smoke-Induced Pulmonary Inflammation and Autophagy Are Attenuated in Ephx2-Deficient Mice. Inflammation. 2017; 40(2):497–510.

23. D'hulst AI, Vermaelen KY, Brusselle GG, Joos GF, Pauwels RA. Time course of cigarette smoke-induced pulmonary inflammation in mice. Eur Respir J. 2005; 26(2):204–213.
24. O'Donnell R, Breen D, Wilson S, Djukanovic R. Inflammatory cells in the airways in COPD. Thorax. 2006; 61(5):448–454.
25. Rovina N, Koutsoukou A, Koulouris NG. Inflammation and immune response in COPD: where do we stand? Mediators Inflamm. 2013; 2013:413735.

26. Karimi K, Sarir H, Mortaz E, Smit JJ, Hosseini H, De Kimpe SJ, Nijkamp FP, Folkerts G. Toll-like receptor-4 mediates cigarette smoke-induced cytokine production by human macrophages. Respir Res. 2006; 7:66.

27. Teasdale JE, Hazell GG, Peachey AM, Sala-Newby GB, Hindmarch CC, McKay TR, Bond M, Newby AC, White SJ. Cigarette smoke extract profoundly suppresses TNFα-mediated proinflammatory gene expression through upregulation of ATF3 in human coronary artery endothelial cells. Sci Rep. 2017; 7:39945.

28. Hubeau C, Kubera JE, Masek-Hammerman K, Williams CM. Interleukin-6 neutralization alleviates pulmonary inflammation in mice exposed to cigarette smoke and poly(I:C). Clin Sci (Lond). 2013; 125(10):483–493.

29. Wei J, Xiong XF, Lin YH, Zheng BX, Cheng DY. Association between serum interleukin-6 concentrations and chronic obstructive pulmonary disease: a systematic review and meta-analysis. PeerJ. 2015; 3:e1199.

30. He JQ, Foreman MG, Shumansky K, Zhang X, Akhabir L, Sin DD, Man SF, DeMeo DL, Litonjua AA, Silverman EK, Connett JE, Anthonisen NR, Wise RA, Paré PD, Sandford AJ. Associations of IL6 polymorphisms with lung function decline and COPD. Thorax. 2009; 64(8):698–704.

31. Malerba M, Radaeli A, Olivini A, Damiani G, Ragnoli B, Montuschi P, Ricciardolo FL. Exhaled nitric oxide as a biomarker in COPD and related comorbidities. Biomed Res Int. 2014; 2014:271918.

32. Hesslinger C, Strub A, Boer R, Ulrich WR, Lehner MD, Braun C. Inhibition of inducible nitric oxide synthase in respiratory diseases. Biochem Soc Trans. 2009; 37(Pt 4):886–891.

33. Agustí A, Morlá M, Sauleda J, Saus C, Busquets X. NF-kappaB activation and iNOS upregulation in skeletal muscle of patients with COPD and low body weight. Thorax. 2004; 59(6):483–487.
34. Wright JL, Zhou S, Churg A. Pulmonary hypertension and vascular oxidative damage in cigarette smoke exposed eNOS(-/-) mice and human smokers. Inhal Toxicol. 2012; 24(11):732–740.
35. Li XQ, Cai LM, Liu J, Ma YL, Kong YH, Li H, Jiang M. Liquiritin suppresses UVB-induced skin injury through prevention of inflammation, oxidative stress and apoptosis through the TLR4/MyD88/NF-κB and MAPK/caspase signaling pathways. Int J Mol Med. 2018; 42(3):1445–1459.

36. Guan Y, Li FF, Hong L, Yan XF, Tan GL, He JS, Dong XW, Bao MJ, Xie QM. Protective effects of liquiritin apioside on cigarette smoke-induced lung epithelial cell injury. Fundam Clin Pharmacol. 2012; 26(4):473–483.

37. Xiong Y, Wang J, Yu H, Zhang X, Miao C, Ma S. The effects of nodakenin on airway inflammation, hyper-responsiveness and remodeling in a murine model of allergic asthma. Immunopharmacol Immunotoxicol. 2014; 36(5):341–348.

38. Ram A, Mabalirajan U, Das M, Bhattacharya I, Dinda AK, Gangal SV, Ghosh B. Glycyrrhizin alleviates experimental allergic asthma in mice. Int Immunopharmacol. 2006; 6(9):1468–1477.

39. Cai X, Wang X, Li J, Chen S. Protective effect of glycyrrhizin on myocardial ischemia/reperfusion injury-induced oxidative stress, inducible nitric oxide synthase and inflammatory reactions through high-mobility group box 1 and mitogen-activated protein kinase expression. Exp Ther Med. 2017; 14(2):1219–1226.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download