Abstract
Serum levels of the pro-inflammatory apolipoprotein CIII (apoCIII) are increased in type-1 diabetic (T1D) patients and when β-cells are exposed to apoCIII they undergo apoptosis, which can be prevented by an antibody against apoCIII. We have previously investigated the BB rat, an animal model that develops a human-like T1D at the age of around 60 days, and found that apoCIII was also increased in sera from pre-diabetic rats and this promoted β-cell death. Lowering apoCIII with an oligonucleotide antisense during a phase of the pre-diabetic period prolonged the time to onset of T1D. In order to find other ways to lower apoCIII we in this study tested non-alcoholic red wine with medium and high concentrations of polyphenols and the lipid-lowering drug, fenofibrate, both reported to decrease the expression of apoCIII by activating peroxisome proliferator-activated receptors. Pre-diabetic BB-rats were treated orally for one month prior to the expected onset of diabetes with the two different wines or fenofibrate. None of the treatments prevented or prolonged the time to onset of diabetes and the expression of apoCIII was unaffected in this animal model for T1D. However, it must be emphasized that this does not exclude that other species can show a response to these substances.
Increased levels of apolipoprotein CIII (apoCIII) in sera from patients with type-1 diabetes mellitus (T1D) have been shown to promote Ca2+-induced β-cell apoptosis [1]. Although the exact molecular mechanisms are not known, we have demonstrated that apoCIII hyperactivates the voltage-gated L-type Ca2+-channel through scavenger-receptor class BI (SR-BI)/β1 integrin-dependent coactivation of PKA and Src [2].
The diabetes-prone BioBreeding (DP-BB) rat colony was established in the 1970s in Ottawa, Canada from outbred Wistar rats that spontaneously developed a human-like autoimmune T1D. Both sexes of DP-BB rats develop pancreatic insulitis, morphologically similar to that observed in human T1D, with a selective destruction of the insulin-secreting β-cells. In our breeding colony DP-BB rats become diabetic when they are around 60 days old.
The pre-diabetic rats have, as human T1D patients, increased serum concentration of apoCIII [3]. We have decreased the endogenous levels of apoCIII by antisense treatment between the age of 12 to 40 days, when they are still in the pre-diabetic phase, and that significantly prolonged the time to onset of diabetes [3].
ApoCIII is an 8.8 kDa polypeptide mainly synthesized in the liver. There are several pathways so far known to be involved in the regulation of apoCIII gene expression. Interestingly, the gene for apoCIII is inversely regulated by insulin, i.e. insulin inhibits transcription while insulin resistance, seen in many type-2 diabetes (T2D) patients, leads to an increase in apoCIII [45]. Other regulators are peroxisome proliferator-activated receptors (PPARs) that reduce apoCIII gene expression [6].
There are several studies where the beneficial effects of polyphenols in wine have been discussed [78] including data showing a decrease in liver mRNA levels of apoCIII [9]. ApoCIII is pro-inflammatory and polyphenol-rich grape products have been suggested to increase the expression or activation of PPARs that antagonize inflammatory transcription factors [8]. There are data showing that streptozotocin-induced diabetic rats have a reduced anti-oxidant capacity, compared to normal rats, which could be restored by administration of polyphenol enriched Chardonnay white wine. The effect was alcohol-independent [10].
Fibrates are used in the treatment of hyperlipidemia and have, among several other actions, been reported to have anti-inflammatory effects and to reduce apoCIII through the activation of PPARα [1112]. There are emerging data that PPARα is also of importance for diabetes induced microvascular complications like retinopathy [1112].
The aim of this study, based on the data that the proinflammatory apoCIII is increased in diabetes [1314], was to investigate if treatments with polyphenol-rich red wine or fenofibrate have any influence on the onset of diabetes in our animal model for T1D, the BB-rat. These animals develop diabetes within a very narrow time window which gives unique opportunities to study the impact of pre-diabetic interventions.
Diabetes Prone Bio Breeding (DP-BB) rats were obtained from our breeding colony. The incidence of diabetes among our rats is 100% with a mean age of onset at 60 days. Diabetes onset is defined by a glucose level of 270 mg/dL or above. The animals were housed under SPF (Specific Pathogen Free) conditions in a temperature- and humidity-controlled room with 12 hours light: dark cycles. They were fed the R36 diet and water ad libitum. All experiments were carried out according to the local Animal Experiment Ethics Committees.
Rats were administered 3.2 mL/kg of red wine (equal to one glass of wine/day) by oral gavage. The treatment was given for one month and started when the rats were 30 days old. Two different kinds of wine were used; Sonovino Primitivo, Italy consisting of 90% Primitivo grapes and 10% Negroamaro containing medium amounts of polyphenols and Reserve du Vieux Noir, Malbec, France made of Malbec grapes containing high levels of polyphenols.
To rule out an effect of alcohol rats received wine where the alcohol had been removed by evaporation, subjecting the wine to 50℃ for 30 min. Control rats were given water by oral gavage.
Rats were administered a daily dose of 100 mg/kg Fenofibrate (Lipanthyl, Abbott, France) dissolved in 1% Methyl cellulose solution (MTC) by oral gavage. The treatment was given for one month and started when the rats were 30 days old. Controls were given the vehicle.
In both treatment groups rats were weighed each day until onset of diabetes. If a rat developed diabetes before the age of 60 days treatment was terminated. Blood samples were taken before and after 30 days of treatment, and at the debut of diabetes.
Total RNA was isolated using the RNeasy Mini Kit according to the manufactures protocol (Qiagen, Germantown, MD, USA). Total RNA was reverse transcribed at 37℃ using the High Capacity cDNA Reverse Transcription kit (Life technologies, Carlsbad, CA, USA). The expression of all genes was measured by real-time quantitative PCR with Maxima SYBR Green qPCR Master Mix with ROX (Thermo scientific, Waltham, MA, USA) on a QuantStudio 5 instrument (Life technologies). β-Actin was used as an endogenous control. Primer sequences will be provided upon request.
Statistical analyzes were performed with GraphPad Prism. For individual experiment, the number of animals used (n) is included in each figure legend in parenthesis. All results are expressed as mean±SEM. A student's t-test or one-way ANOVA (Tukey's post-hoc) were used when appropriate. P values <0.05 were considered statistically significant, however, this level was not reached in any of the experiments.
The BB-rat animal model for T1D is suitable for intervention studies as the onset of diabetes appears within a narrow time window around 60 days of age and all DP-BB rats in our colony develop the disease. We have previously shown that lowering apoCIII by antisense treatment during the prediabetic phase prolongs the time to onset of disease [3]. In this study we wanted to examine whether red wine containing polyphenols or the lipid-lowering drug, fenofibrate, both reported to decrease apoCIII, have any effect on the debut of diabetes.
In the wine experiments the rats received a daily dose of red wine equivalent to one standard glass. The wine, and the water to the control rats, were given by oral gavage to ensure that they all got the same dose. Two brands of wine with different contents of polyphenols were tested. The animals were treated for one month, but we could not see any effect on the onset of disease by the different wines (Figure 1A). Body weight did not differ significantly, although there seemed to be a tendency to lower weight in both wine groups (Figure 1B).
In the second experiment fenofibrate, that is mainly used to treat hypertriglyceridemia, was given once per day and, as with the wine, it was administered by oral gavage. Neither did we see any effects of this treatment. The age at onset of T1D and body weight were similar in rats treated with fenofibrate as to those receiving the vehicle (Figure 2A, B).
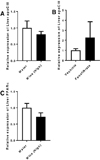
Since our hypothesis was that polyphenols and fenofibrate, by reducing the levels of the pro-inflammatory lipoprotein apoCIII will affect the onset of diabetes, the expression levels of apoCIII were analyzed in liver samples, as liver is the main source of apoCIII. None of the treatments lowered the expression of the lipoprotein (Figure 3A, B). As polyphenols have been reported to up-regulate the expression of PPARα, while fenofibrate activates the receptor, the expression of PPARα was analysed in livers from rats that had been given wine with high concentration of polyphenols, but the levels were similar to those given water (Figure 3C).
ApoCIII is well-known within the field of cardiovascular research, where increased levels have been demonstrated to be pro-inflammatory resulting in atherosclerosis and higher risk for cardiovascular diseases (CVD) [1516]. It has convincingly been shown in both human and animal studies that there is a connection between apoCIII, hypertriglyceridemia and CVD [1718].
ApoCIII has not only been related to CVD, but also to diabetes. It has been demonstrated that haplotypes in the apoCIII gene leading to augmented levels of apoCIII are associated with an increased susceptibility to T1D [19]. Furthermore, apoCIII gene variants with increased levels of apoCIII are associated with the development of non-alcoholic fatty liver disease (NAFLD), hepatic insulin resistance and T2D [2021]. On the contrary, there are humans with mutations in the apoCIII gene leading to life-long reduced levels of apoCIII. These individuals are healthier with favorable pattern of lipoproteins, increased insulin sensitivity, lower incidence of hypertension and they live longer [222324]. Hence, there are a multitude of beneficial health effects lowering apoCIII.
There are several factors involved in development of T1D. We have previously demonstrated that sera from patients with T1D contain increased levels of apoCIII and that this promotes Ca2+-induced β-cell apoptosis that can be prevented by reducing apoCIII [13]. That increased levels of apoCIII are indeed of importance for β-cell function and survival was demonstrated in vivo in the T1D BB rat animal model. When apoCIII was lowered by antisense during a phase of the pre-diabetic period the time to onset of diabetes was prolonged by comparable eight human years [3].
ApoCIII has also been shown to be increased in patients with type-2 diabetes (T2D) [2526] and it is known that insulin resistance, as well as deficiency of insulin, up-regulates the apoCIII gene [45].
During many years there have been discussions whether a moderate consumption of red wine is beneficial. It has for example been shown that red wine rich in polyphenols with and without alcohol improves insulin sensitivity and activates anti-inflammatory and anti-oxidant processes with positive effects on CVD [2728]. Furthermore, the use of lipid lowering substances has been reported not only to reduce the risk of CVD by lowering lipids, but also to have anti-inflammatory effects [29]. Both polyphenols and fibrate have been reported, among a lot of other effects, to lower apoCIII. Since it would be desirable to use an alternative to antisense to decrease apoCIII, we decided to investigate if the onset of T1D in our BB rat animal model could be affected by treating with polyphenol containing wine or fibrate.
We tested two red wines (without alcohol) with different content of polyphenols and fenofibrate, but neither of the treatments had any influence on the debut of T1D. When measuring the expression levels of apoCIII there was no difference which is in line with the observation that the onset of T1D was unaffected.
Rats treated with wine had a tendency to a lower body weight. This has been observed in other studies that a moderate consumption of wine decreases weight and even partially prevents high-fat diet induced weight gain [3031].
In a study in Sprague-Dawley rats, 2 and 24- months old, treated with fenofibrate for two weeks, an age-related reduced expression of PPARα was seen, but no effect by fenofibrate although there was a decrease in triglycerides showing that the drug was absorbed [32]. However, apoCIII mRNA levels were reduced. The authors suggested that this could reflect a functional activation of PPARα despite no change in expression.
There could be several explanations why there is a discrepancy to our results. Important differences are the age of the animals, the treatment time and the use of a different strain, the diabetes-prone BB rat is on a Wistar background. Furthermore, the Sprague-Dawley rats were not in a pre-diabetic stage where many different pathways can be affected, prior to the onset of disease, influencing the responses.
In conclusion, we demonstrate that neither red wine nor fenofibrate lowered apoCIII or affected the onset of diabetes in the BB rat T1D animal model. However, it should be emphasized that this does not exclude that other species may show a different response to these substances. Since apoCIII is regulated by several different pathways it might be necessary to lower it either at the transcription level with antisense, as we have shown before [3], or at the protein level by inhibiting the function using an antibody, nanobody or aptamer against apoCIII.
Figures and Tables
Figure 1
Age at diabetes onset and body weight at the end of the treatments. Pre-diabetic rats were treated with water (white), non-alcohol containing red wine with medium (black) or high (grey) levels of polyphenols from 30–60 days of age. A) Age at onset of diabetes. B) Body weight at the end of the treatments. Data are presented as mean SEM (n= 9, 3 and 5, respectively).

Figure 2
Age at diabetes onset and body weight at the end of the treatment with fenofibrate. Pre-diabetic rats were treated with vehicle (white) or a daily dose of 100 mg/kg fenofibrate (black) from 30 to 60 days of age. A) Age at onset of diabetes. B) Body weight at the end of the treatment. Data are presented as mean SEM (n=4).
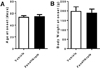
Figure 3
Expression of apoCIII and PPARα in liver. Expression of apoCIII after treatment with A) water (white) or wine with high content of polyphenols (black) and B) vehicle (white) or fenofibrate (black). C) Expression of PPARα after treatment with water (white) or wine with high content of polyphenols (black). Data are presented as mean SEM (n=3–4)
Acknowledgments
This work was supported by the Swedish Diabetes Association, Funds of Karolinska Institutet, The Swedish Research Council, Novo Nordisk Foundation, The Family Erling-Persson Foundation, Strategic Research Program in Diabetes at Karolinska Institutet, The Family Knut and Alice Wallenberg Foundation, Skandia Insurance Company, Ltd., Diabetes and Wellness Foundation, The Stichting af Jochnick Foundation, The Bert von Kantzow Foundation, Svenska Diabetesstiftelsen, AstraZeneca, Swedish Association for Diabetology and The ERC-2013-AdG 338936-BetaImage.
References
1. Juntti-Berggren L, Larsson O, Rorsman P, Ammälä C, Bokvist K, Wåhlander K, Nicotera P, Dypbukt J, Orrenius S, Hallberg A, Berggren PO. Increased activity of L-type Ca2+ channels exposed to serum from patients with type I diabetes. Science. 1993; 261(5117):86–90.

2. Shi Y, Yang G, Yu J, Yu L, Westenbroek R, Catterall WA, Juntti-Berggren L, Berggren PO, Yang SN. Apolipoprotein CIII hyperactivates β cell CaV1 channels through SR-BI/β1 integrin-dependent coactivation of PKA and Src. Cell Mol Life Sci. 2014; 71(7):1289–1303.

3. Holmberg R, Refai E, Höög A, Crooke RM, Graham M, Olivecrona G, Berggren PO, Juntti-Berggren L. Lowering apolipoprotein CIII delays onset of type 1 diabetes. Proc Natl Acad Sci U S A. 2011; 108(26):10685–10689.

4. Chen M, Breslow JL, Li W, Leff T. Transcriptional regulation of the apoC-III gene by insulin in diabetic mice: correlation with changes in plasma triglyceride levels. J Lipid Res. 1994; 35(11):1918–1924.

5. Altomonte J, Cong L, Harbaran S, Richter A, Xu J, Meseck M, Dong HH. Foxo1 mediates insulin action on apoC-III and triglyceride metabolism. J Clin Invest. 2004; 114(10):1493–1503.

6. Vu-Dac N, Gervois P, Torra IP, Fruchart JC, Kosykh V, Kooistra T, Princen HM, Dallongeville J, Staels B. Retinoids increase human apo C-III expression at the transcriptional level via the retinoid X receptor. Contribution to the hypertriglyceridemic action of retinoids. J Clin Invest. 1998; 102(3):625–632.

7. Palsamy P, Subramanian S. Ameliorative potential of resveratrol on proinflammatory cytokines, hyperglycemia mediated oxidative stress, and pancreatic beta-cell dysfunction in streptozotocin-nicotinamide-induced diabetic rats. J Cell Physiol. 2010; 224(2):423–432.
8. Chuang CC, McIntosh MK. Potential mechanisms by which polyphenol-rich grapes prevent obesity-mediated inflammation and metabolic diseases. Annu Rev Nutr. 2011; 31:155–176.

9. Del Bas JM, Fernández-Larrea J, Blay M, Ardèvol A, Salvadó MJ, Arola L, Bladé C. Grape seed procyanidins improve atherosclerotic risk index and induce liver CYP7A1 and SHP expression in healthy rats. FASEB J. 2005; 19(3):479–481.

10. Landrault N, Poucheret P, Azay J, Krosniak M, Gasc F, Jenin C, Cros G, Teissedre PL. Effect of a polyphenols-enriched chardonnay white wine in diabetic rats. J Agric Food Chem. 2003; 51(1):311–318.

11. Noonan JE, Jenkins AJ, Ma JX, Keech AC, Wang JJ, Lamoureux EL. An update on the molecular actions of fenofibrate and its clinical effects on diabetic retinopathy and other microvascular end points in patients with diabetes. Diabetes. 2013; 62(12):3968–3975.

12. Chen Y, Hu Y, Lin M, Jenkins AJ, Keech AC, Mott R, Lyons TJ, Ma JX. Therapeutic effects of PPARα agonists on diabetic retinopathy in type 1 diabetes models. Diabetes. 2013; 62(1):261–272.

13. Juntti-Berggren L, Refai E, Appelskog I, Andersson M, Imreh G, Dekki N, Uhles S, Yu L, Griffiths WJ, Zaitsev S, Leibiger I, Yang SN, Olivecrona G, Jörnvall H, Berggren PO. Apolipoprotein CIII promotes Ca2+-dependent beta cell death in type 1 diabetes. Proc Natl Acad Sci U S A. 2004; 101(27):10090–10094.
14. Åvall K, Ali Y, Leibiger IB, Leibiger B, Moede T, Paschen M, Dicker A, Daré E, Köhler M, Ilegems E, Abdulreda MH, Graham M, Crooke RM, Tay VS, Refai E, Nilsson SK, Jacob S, Selander L, Berggren PO, Juntti-Berggren L. Apolipoprotein CIII links islet insulin resistance to β-cell failure in diabetes. Proc Natl Acad Sci U S A. 2015; 112(20):E2611–E2619.

15. Hiukka A, Ståhlman M, Pettersson C, Levin M, Adiels M, Teneberg S, Leinonen ES, Hultén LM, Wiklund O, Oresic M, Olofsson SO, Taskinen MR, Ekroos K, Borén J. ApoCIII-enriched LDL in type 2 diabetes displays altered lipid composition, increased susceptibility for sphingomyelinase, and increased binding to biglycan. Diabetes. 2009; 58(9):2018–2026.

16. Kohan AB. Apolipoprotein C-III: a potent modulator of hypertriglyceridemia and cardiovascular disease. Curr Opin Endocrinol Diabetes Obes. 2015; 22(2):119–125.
17. Norata GD, Tsimikas S, Pirillo A, Catapano AL. Apolipoprotein C-III: From Pathophysiology to Pharmacology. Trends Pharmacol Sci. 2015; 36(10):675–687.

18. Ooi EM, Barrett PH, Chan DC, Watts GF. Apolipoprotein C-III: understanding an emerging cardiovascular risk factor. Clin Sci (Lond). 2008; 114(10):611–624.

19. Hokanson JE, Kinney GL, Cheng S, Erlich HA, Kretowski A, Rewers M. Susceptibility to type 1 diabetes is associated with ApoCIII gene haplotypes. Diabetes. 2006; 55(3):834–838.

20. Petersen KF, Dufour S, Hariri A, Nelson-Williams C, Foo JN, Zhang XM, Dziura J, Lifton RP, Shulman GI. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. N Engl J Med. 2010; 362(12):1082–1089.

21. Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014; 510(7503):84–91.

22. Pollin TI, Damcott CM, Shen H, Ott SH, Shelton J, Horenstein RB, Post W, McLenithan JC, Bielak LF, Peyser PA, Mitchell BD, Miller M, O'Connell JR, Shuldiner AR. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008; 322(5908):1702–1705.

23. Atzmon G, Rincon M, Schechter CB, Shuldiner AR, Lipton RB, Bergman A, Barzilai N. Lipoprotein genotype and conserved pathway for exceptional longevity in humans. PLoS Biol. 2006; 4(4):e113.

24. Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014; 371(1):32–41.
25. Alaupovic P, Bard JM, Tavella M, Shafer D. Identification of apoB-containing lipoprotein families in NIDDM. Diabetes. 1992; 41:Suppl 2. 18–25.

26. Florez H, Mendez A, Casanova-Romero P, Larreal-Urdaneta C, Castillo-Florez S, Lee D, Goldberg R. Increased apolipoprotein C-III levels associated with insulin resistance contribute to dyslipidemia in normoglycemic and diabetic subjects from a triethnic population. Atherosclerosis. 2006; 188(1):134–141.

27. Chiva-Blanch G, Urpi-Sarda M, Ros E, Valderas-Martinez P, Casas R, Arranz S, Guillén M, Lamuela-Raventós RM, Llorach R, Andres-Lacueva C, Estruch R. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: a randomized clinical trial. Clin Nutr. 2013; 32(2):200–206.

28. Markoski MM, Garavaglia J, Oliveira A, Olivaes J, Marcadenti A. Molecular Properties of Red Wine Compounds and Cardiometabolic Benefits. Nutr Metab Insights. 2016; 9:51–57.

29. Muhlestein JB, May HT, Jensen JR, Horne BD, Lanman RB, Lavasani F, Wolfert RL, Pearson RR, Yannicelli HD, Anderson JL. The reduction of inflammatory biomarkers by statin, fibrate, and combination therapy among diabetic patients with mixed dyslipidemia: the DIACOR (Diabetes and Combined Lipid Therapy Regimen) study. J Am Coll Cardiol. 2006; 48(2):396–401.
30. Vadillo M, Ardévol A, Fernández-Larrea J, Pujadas G, Bladé C, Salvadó MJ, Arola L, Blay M. Moderate red-wine consumption partially prevents body weight gain in rats fed a hyperlipidic diet. J Nutr Biochem. 2006; 17(2):139–142.
31. Milat AM, Mudniæ I, Grkoviæ I, Kljuèeviæ N, Grga M, Jerèiæ I, Juriæ D, Ivankoviæ D, Benzon B, Boban M. Effects of White Wine Consumption on Weight in Rats: Do Polyphenols Matter? Oxid Med Cell Longev. 2017; 2017:8315803.

32. Ye P, Wang ZJ, Zhang XJ, Zhao YL. Age-related decrease in expression of peroxisome proliferator-activated receptor alpha and its effects on development of dyslipidemia. Chin Med J (Engl). 2005; 118(13):1093–1098.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download