Abstract
The butanol extract of Asparagus cochinchinensis roots fermented with Weissella cibaria (BAfW) significantly suppressed the inflammatory response induced by lipopolysaccharide (LPS) treatment in RAW264.7 cells. To investigate the dose dependence and durability of BAfW on the anti-asthma effects, alterations in key parameters were measured in ovalbumin (OVA)-challenged Balb/c mice treated with the different doses of BAfW at three different time points. The number of immune cells, OVA-specific IgE level, thickness of respiratory epithelium and mucus score decreased significantly in a dose-dependent manner in response to treatment with 125 to 500 mg/kg BAfW (P<0.05), although the highest level was detected in the 500 mg/kg treated group. Moreover, the decrease in these parameters was maintained from 24 to 48 h in the 500 mg/kg of BAfW treated group. At 72 h, the effects of BAfW on the number of immune cells, OVA-specific IgE level and thickness of respiratory epithelium partially disappeared. Overall, this study provides the first evidence that the anti-asthma effect of BAfW may reach the maximum level in OVA-challenged Balb/c mice treated with 500 mg/kg and that these effects can last for 48 h.
Asthma is defined as a chronic airway inflammatory disease characterized by airway remodeling and hyperresponsiveness, mucus hyperproduction, and infiltration of the airway by mast cells, eosinophils and T helper 2 (Th2) cells [123]. This disease has commonly been treated with steroids (inhaled, oral or by injection) to help calm airway inflammation [4]. However, steroids have adverse effects such as cataracts and glaucoma, hypertension, hyperlipidemia, peptic ulcers, myopathy, immunosuppressive effects and growth inhibition [56]. Therefore, various natural products have received attention because of their potential to overcome these limitations. Significant anti-asthmatic activity was detected in the ethanol extract of Saururus chinensis [7], the polyphenolic extracts of Laurencia undulata [8] and schizandrin [9], the ethanol extract of Sanguisorba officinalis L. [10], and the ethanoic extracts of Angelica dadurica Bentham et Hooker [11] and saponin-enriched extract of Asparagus cochinchinensis [12] following their application in OVA-challenged asthma models. Similar effects were observed in milk-induced asthma mice models treated with Solanum xanthocarpum flower extract [13] and methanol extract of Tamarindus indica L. [14], as well as a guinea pig model treated with Perilla seed oil [15]. However, no studies have investigated the dose dependence and durability of natural products with anti-asthma activity.
The root extracts of A. cochinchinensis are known to have anti-inflammatory activity, and several of their extracts were found to significantly inhibit the expression of inflammatory cytokines, NO production and the progression of cutaneous inflammation in LPS-stimulated mouse astrocytes and BV-2 microglial cells, as well as in animal models treated with 12-O-tetradecanoylphorbol-13-acetate (TPA) [16]. Other extracts suppressed the skin inflammation induced by phthalic anhydride (PA) treatment and airway inflammation in the lung tissue of the OVA-challenged asthma model [171819]. Furthermore, their fermented product, BAfW, suppressed the iNOS-mediated COX-2 induction pathway and expression of inflammatory cytokines in LPS-activated RAW264.7 cells. BAfW contained an enhanced concentration of protodioscin (9.3%) and a steroidal saponin compound (126.6%) compared with unfermented products. Also, the protodioscin was detected as one of indicators in BAfW [20]. However, no studies have investigated the dose dependence and durability of BAfW in OVA-challenged Balb/c mice to evaluate their potential for use as an anti-asthmatic drug.
Therefore, this study was conducted to investigate the anti-asthmatic effects of BAfW based on dose dependence and their ability to prevent airway inflammation and remodeling activity using OVA-challenged Balb/c mice. The results presented herein provide important information regarding therapeutic dose and duration of BAfW that will be useful to future investigations of the therapeutic effects and action mechanism of BAfW.
Freeze-dried roots of A. cochinchinensis (20 g) were ground to a powder, after which hot water extract was prepared by mixing with 1.2 L of deionized distilled water (dH2O) and then placing the mixture in a hot water extractor (DW-790, Daewoong, Hwaseong, Korea) for 2.5 h. Following aqueous extraction, the samples were filtered through Whatman No. 2 filter paper (Whatman, Brentford, UK), after which they were evaporated in a rotary vacuum evaporator (EYELA, N-1100 series, Tokyo, Japan) and lyophilized. This freeze-dried, unfermented A. cochinchinensis root (UnFAR) powder was used for fermentation. The extraction yield in hot water was 60.7% [12].
The bacterial strains of W. cibaria used in the fermentation process were provided by Professor Hong Joo Son, Department of Life Science and Environmental Biochemistry, Pusan National University. To prepare the fermented products, UnFAR powder was dissolved at 1% (w/v) in dH2O (pH 5.3), then sterilized at 121℃ for 15 min. After cooling to room temperature, the UnFAR mixture solution was inoculated with W. cibaria [5% (v/v)] that had been precultivated in lactobacilli MRS broth (Difco Laboratories, Detroit, USA) to a final cell density of 107 CFU/mL (OD600=0.1). The mixture was then incubated in a shaking incubator (VS-8480, Vision Scientific, Bucheon, Korea) at 37℃ and 200 rpm for 4.3 days. After fermentation with W. cibaria, the mixture was centrifuged at 12,000×g for 10 min to obtain the fermented A. cochinchinensis products of W. cibaria (FARW).
To obtain the n-butanol fractions of FARW (BAfW), an equal volume of butanol was added to the FARW. After vigorous mixing followed by incubation, the butanol phase was collected from each mixture by centrifugation at 12,000×g for 10 min. The butanol extraction was repeated three times, after which all butanol phases were combined, evaporated with a rotary vacuum evaporator, freeze-dried, and stored at −20℃ until further use. Finally, the collected BAfW powder was dissolved in 0.5% Tween-20 solution in distilled water (dH2O) to 500 mg/kg, and then further diluted to the required concentration.
The animal protocols for our study were reviewed and approved for ethical and scientific care procedures by the Pusan National University-Institutional Animal Care and Use Committee (PNU-IACUC; Approval Number PNU-2015-0976). Six-week-old Balb/c mice (female) were purchased from Samtako BioKorea Co. (Osan, Korea). Before starting the animal experiment, the mice allowed to acclimatize to the experimental environment for at least 1 week. All mice were provided with ad libitum access to a standard irradiated chow diet (Samtako BioKorea Co.) and water throughout the experimental period. During the experiment, mice were maintained in a specific pathogen-free state under a strict light cycle (lights on at 08:00 h and off at 20:00 h) at 23±2℃ and 50±10% relative humidity. Balb/c mice were housed at the Pusan National University-Laboratory Animal Resources Center accredited by the Korea Food and Drug Administration (FDA) (Accredited Unit Number: 000231) and AAALAC International (Accredited Unit Number: 001525).
The airway challenge of Balb/c mice was conducted as previously described [2122]. To analysis dose dependence, 6-week-old Balb/c mice (female, n=70) were assigned to either a No treated group (n=7) or OVA treated group (n=63). The No treated group remained untreated during the experimental period. OVA-challenged asthma was generated by sensitization for 20 days, then challenged for 3 days. At day 1 and day 14, all mice were sensitized by intraperitoneal injection with OVA (20 µg) (albumin from chicken, Sigma-Aldrich Co., St. Louis, USA) emulsified with aluminum hydroxide (Alum, Sigma-Aldrich Co.) in 200 µL 1×PBS solution. At days 21–23, the exposed mice were subjected to a 30 minute airway challenge with 2% OVA in 1×PBS that was administered by inhalation through a nebulizer (Omron, Tokyo, Japan). The OVA-challenged asthma group was further divided into a vehicle treated group (OVA+Vehicle, n=7) and BAfW treated group (OVA+BAfW, n=7 per each group). The nine different concentrations (2, 3.9, 7.8, 15.6, 31.3, 62.5, 125, 250 and 500 mg/kg body weight) of BAfW were orally administered into one of OVA+BAfW treated groups, whereas the same volume of 0.5% Tween-20 solution was treated into the OVA+Vehicle group. At 48 h after the final treatment, all animals were euthanized using CO2 gas, and tissue samples were acquired and stored in Eppendorf tubes at −70℃ until assay.
Analysis for durability was conducted using the same methods, but different treatment doses of BAfW and sacrifice times after the final treatment. During these processes, the OVA+BAfW treated groups received only 500 mg/kg body weight of BAfW for 6 days. At 24, 48, and 72 h after the final treatment, all animals were euthanized using CO2 gas, and tissue samples were collected for further analysis.
After the Balb/c mice were anaesthetized with alfaxalone (Alfaxan, Hunter Valley, Australia), BALF was obtained (yield: 80%, total volume of 0.8 mL) from subset groups via tracheal cannulation using cold 1×PBS. Total cells were harvested from BALF by centrifugation (2,000×g) at 4℃ for 5 min, after which they were re-suspended in 100 µL of hematoxylin or May-Giemsa solution (Sigma-Aldrich Co.). The eosinophils, macrophages and total cells were counted on glass slides using the Leica Application Suite (Leica Microsystems, Wetzlar, Germany).
The OVA-specific IgE concentration in the BALF of mice was measured using an Mouse OVA-specific IgE ELISA kit (BioLegend Inc., San Diego, USA), according to the manufacturer's instructions. Briefly, wells were washed with washing solution (50 mM Tris, 0.14 M NaCl, 0.05% Tween 20, pH 8.0) four times, after which assay buffer (50 µL) and BALF (50 µL) were added to wells coated with antibody. The plates were then incubated for 2 h with shaking at room temperature, after which the wells were washed with washing solution. Next, 100 µL of detection antibody solution was added per well, and the samples were incubated for 1 h with shaking at room temperature. After washing the wells, Avidin-HRP D solution (100 µL) was added to each well. The plates were then incubated at room temperature for 30 min, washed, and an enzyme reaction was initiated by adding the substrate solution. The plates were subsequently incubated in the dark at room temperature for 15 min, after which the reaction was terminated by adding 2M H2SO4 solution and the absorbance was measured at 450 nm using a Versa-max plate reader (Molecular Devices, Sunnyvale, USA).
Briefly, lung tissues were collected from all OVA-treated Balb/c mice of subset groups, fixed in 10% neutral buffered formalin, embedded in paraffin wax, processed routinely, and sectioned into 4 µm thick slices. The tissue sections were then collected on slide glass and stained with hematoxylin and eosin (H&E) (IHC World, Woodstock, USA), after which they were examined by light microscopy at 400× magnification for infiltration of inflammatory cells into the peribronchial region of the lung. The epithelial thickness of the bronchial tube was also measured using the Leica Application Suite (Leica Microsystems).
Goblet cell hyperplasia for mucus production was detected by staining with periodic acid-schiff (PAS). Following deparaffinization and dehydration of the lung sections, samples were oxidized in periodic acid solution for 5 min. The lung sections were then washed and placed in Schiff reagent for 15 min. Next, samples were washed with warm tap water, stained with hematoxylin solution for 30 seconds (IHC World), and then examined for mucus score by light microscopy at 400× magnification.
One-way ANOVA was used to identify significant differences between the OVA+Vehicle treated group and the OVA+BAfW treated group (SPSS for Windows, Release 10.10, Standard Version, Chicago, IL, USA). These levels were verified by Tukey's post-hoc analysis. All values were expressed as the means±SD and a P value <0.05 was considered significant.
To investigate the dose dependence of BAfW in an OVA-challenged asthma model, alterations in the number of immune cells, IgE concentration and histopathological structure were measured in OVA induced Balb/c mice treated with nine different concentrations (from 2 to 500 mg/kg) of BAfW. The total number of cells and the IgE concentration in BALF gradually decreased in a dose-dependent manner in the OVA+Vehicle treated group. However, these decrease were only statistically significant for the 125–500 mg/kg BAfW treated group (for total cells) and the 31.3–500 mg/kg BAfW treated group (for OVA-specific IgE concentration) (Figures 1A and B).
Similar patterns in the above parameters were detected upon analysis of the histological structure and mucin secretion of the airways. The thickness of the respiratory epithelium and the mucus score in lung tissue sections were significantly decreased in the 125–500 mg/kg BAfW treated group (P<0.05) compared to the OVA+Vehicle treated group (Figures 2 and 3). These results suggest that the significant therapeutic effects of BAfW could be detected at 125–500 mg/kg in the OVA-challenged asthma model, although the anti-asthma effect increased with the dose of BAfW.
To determine the duration of BAfW effect in an OVA-challenged asthma model, alterations in the number of immune cells, IgE concentration and histopathological structure were measured in the OVA-challenged Balb/c mice treated with 500 mg/kg of BAfW at three different time points (24, 48, and 72 h) after the final treatment. The total number of cells and the IgE concentration in BALF decreased gradually in a time-dependent manner until 48 h in the OVA+BAfW treated group. However, in the group treated with OVA+BAfW for 72 h, these levels increased slightly to the same level as in the group treated with OVA+BAfW for 24 h (Figure 4). A similar pattern was also observed in the thickness of the respiratory epithelium of the OVA+BAfW treated group (Figure 5). However, airway mucin secretion showed a different pattern. Specifically, the mucus score in lung tissue sections gradually decreased over 72 h in the OVA+BAfW treated groups when compared to the OVA+Vehicle treated group. The lowest mucus score was detected in the group treated with OVA+BAfW for 72 h (Figure 6). Therefore, these results suggest that the therapeutic effects of BAfW lasted for 72 h in the OVA-induced asthma model, although there was slight variation in the magnitude of the anti-asthma reaction.
Pharmacokinetics (PK) and pharmacodynamics (PD) are important to modern drug development because the therapeutic and toxic effects of natural products are tightly related to their concentrations at the target site [23]. Therefore, many studies have investigated the dose dependence and durability of natural products during in vivo studies as an early step in the drug development process. In this study, we analyzed the therapeutic effects of BAfW based on the treated dose and elapsed time after final treatment in an OVA-challenged asthma model. The results presented herein provide basic information that will be useful to future investigations of the action mechanism of BAfW as well as to establishment of an experimental scheme for PK and PD studies.
The anti-asthma effects of some natural products have been widely investigated at various concentration in several animal model. However, their optimal concentrations for therapeutic effects have not been closely investigated. Most of these studies have revealed excellent therapeutic effects at concentrations less than 300 mg/kg when applied to treat OVA-challenged asthma model. For example, the maximum anti-asthma effects were observed at 300 mg/kg for Actinidia argute water-soluble extract [24], 200 mg/kg of ethanol extract of Saururus chinensis [7], 200 mg/kg of Sanguisorba officinalis L. ethanolic extract [10] and 20mg/kg of Laurencia undulata ethanolic extract [8]. However, few products showed their maximum anti-asthma effects at high concentrations. Perilla seed oil significantly improved lung function in a sensitized guinea pig model treated with 2,000 mg/kg [15], while methanolic extract of the leaves of Tamarindus indica L. exhibited anti-asthmatic adipogenic and mast cell stabilizing activity in milk- or clonidine-induced mouse models treated with 1,000 mg/kg [14]. In our study, the optimal anti-asthma effects of BAfW were detected at 500 mg/kg in an OVA-challenged Balb/c model. This concentration of BAfW was higher than those observed in previous studies using OVA-induced models. However, most parameters indicated that significant anti-asthmatic effects began at a dose of 125 mg/kg of BAfW compared with the OVA+Vehicle treated group. Therefore, the results of the present study are similar to those of previous studies using OVA-challenged models; however, further studies are required to determine the maximum permissible concentration.
The durability of natural products with anti-asthma effects has not been investigated in previous studies; rather, parameters have only been investigated for therapeutic effects at specific time points. For example, the therapeutic effect of Perilla seed oil [15], flower extracts of Solanum xanthocarpum [13] and methanol extract of leaves of Tamarindus indica L. [14] were analyzed at 24 h after the final treatment, while the anti-asthma effects of Laurencia undulata polyphenolic extracts [8], Angelica dahurica [11], schizandrin [9] and Sanguisorba officinalis L. [10] were investigated at 48 h after the final treatment. In the present study, we determined the durability of BAfW on the anti-asthma effects in an OVA-challenged asthma model. We found a different pattern in the level of anti-asthma parameters observed at 72 h. Specifically, the inhibitory effect on the number of macrophages and eosinophils as well as the concentration of OVA-specific IgE began to decrease in the OVA-challenged asthma model treated with BAfW (500 mg/kg). These results provide the first evidence of the durability of BAfW with anti-asthma effects in an OVA-challenged asthma model.
Taken together, this is the first study to examine the dose dependence and durability of BAfW with anti-asthma effects in an OVA-challenged asthma model. In addition, the results presented herein provide evidence that the optimal anti-asthma effects of BAfW could be observed in an OVA-challenged asthma model following treatment with 500 mg/kg of BAfW for 48 h.
Figures and Tables
 | Figure 1Measurement of number of total immune cells and level of OVA-specific IgE in BALF for dose dependence analysis of BAfW. (A) After collection of BALF from the lungs, total cells were separated by centrifugation and stained with May-Giemsa solution. The total number of cells within a 1 mm2 area was counted under a light microscope at 400× magnification. (B) The concentration of OVA-specific IgE was quantified in BALF using an enzyme-linked immunosorbent assay kit with a detection limit of 20.7 pg/mL. Data shown are the means±SD (n=7). * indicates P<0.05 compared to the OVA+Vehicle treated group. |
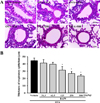 | Figure 2Observation of histopathological structure of lung tissue during dose dependence analysis of BAfW. (A) The bronchial thickness was observed in H&E stained lung tissue at 400× magnification. (B) Respiratory epithelium thickness was measured using the Leica Application Suite. Data shown are the means±SD (n=7). * indicates P<0.05 compared to the OVA+Vehicle treated group. |
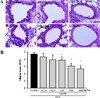 | Figure 3Detection of mucin secretion in lung tissue during dose dependence analysis of BAfW. (A) After staining with Periodic Acid Schiff (PAS), goblet cell hyperplasia was observed in lung tissue. (B) The mucus score was determined by three independent investigators in a single-blind study analysis based on evaluation of four different randomly selected locations using a microscope. 0, no mucus; 1, <5% of the epithelium; 2, 5–10% of the epithelium; 3, 10–20% of the epithelium; 4, 20–30% of the epithelium; 5, 30–40% of the epithelium. Data shown are the means±SD (n=7). * indicates P<0.05 compared to the OVA+Vehicle treated group. |
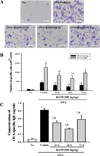 | Figure 4Measurement of number of total immune cells and level of OVA-specific IgE in BALF for durability analysis of BAfW. (A) After collection of BALF from the lungs, total cells were separated by centrifugation and stained with May-Giemsa solution. (B) The number of total cells was then counted within a 1 mm2 area under a light microscope at 400× magnification. (C) The concentration of OVA-specific IgE was quantified in BALF using an enzyme-linked immunosorbent assay kit with a detection limit of 20.7 pg/mL. Data shown are the means±SD (n=7). * indicates P<0.05 compared to the No treated group. # indicates P<0.05 compared to the OVA+Vehicle treated group. |
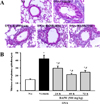 | Figure 5Observation of histopathological structure of lung tissue for durability analysis of BAfW. (A) The bronchial thickness was observed in H&E stained lung tissue at 400× magnification. (B) Respiratory epithelium thickness was measured using the Leica Application Suite. Data shown are the means±SD (n=7). * indicates P<0.05 compared to the No treated group. # indicates P<0.05 compared to the OVA+Vehicle treated group. |
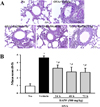 | Figure 6Detection of mucin secretion from lung tissue for durability analysis of BAfW. (A) After staining with Periodic Acid Schiff (PAS), goblet cell hyperplasia was observed in lung tissue. (B) The mucus score was determined by three independent investigators in a single-blind study analysis based on four different randomly selected locations using a microscope. 0, no mucus; 1, < 5% of the epithelium; 2, 5–10% of the epithelium; 3, 10–20% of the epithelium; 4, 20–30% of the epithelium; 5, 30–40% of the epithelium. Data shown are the means±SD (n=7). * indicates P<0.05 compared to the No treated group. # indicates P<0.05 compared to the OVA+Vehicle treated group. |
Acknowledgments
We thank Jin Hyang Hwang for directing the care and use of animals at the Laboratory Animal Resources Center at Pusan National University. This work was supported for two years by a Pusan National University Research Grant.
References
1. Endo Y, Hirahara K, Yagi R, Tumes DJ, Nakayama T. Pathogenic memory type Th2 cells in allergic inflammation. Trends Immunol. 2014; 35(2):69–78.

2. Rosenberg JL. Antilipid agents may provide allergy protection. Ann Allergy Asthma Immunol. 2013; 110(1):1.

3. Porter PC, Yang T, Luong A, Delclos GL, Abramson SL, Kheradmand F, Corry DB. Proteinases as molecular adjuvants in allergic airway disease. Biochim Biophys Acta. 2011; 1810(11):1059–1065.

4. Walsh GM. Targeting airway inflammation: novel therapies for the treatment of asthma. Curr Med Chem. 2006; 13(25):3105–3111.
5. Wise J. Corticosteroids for asthma may suppress growth in children in first year of treatment, researchers say. BMJ. 2014; 349:g4623.

6. Ciriaco M, Ventrice P, Russo G, Scicchitano M, Mazzitello G, Scicchitano F, Russo E. Corticosteroid-related central nervous system side effects. J Pharmacol Pharmacother. 2013; 4:Suppl 1. S94–S98.

7. Lee E, Haa K, Yook JM, Jin MH, Seo CS, Son KH, Kim HP, Bae KH, Kang SS, Son JK, Chang HW. Anti-asthmatic activity of an ethanol extract from Saururus chinensis. Biol Pharm Bull. 2006; 29(2):211–215.
8. Jung WK, Choi I, Oh S, Park SG, Seo SK, Lee SW, Lee DS, Heo SJ, Jeon YJ, Je JY, Ahn CB, Kim JS, Oh KS, Kim YM, Moon C, Choi IW. Anti-asthmatic effect of marine red alga (Laurencia undulata) polyphenolic extracts in a murine model of asthma. Food Chem Toxicol. 2009; 47(2):293–297.
9. Lee MY, Seo CS, Lee NH, Ha H, Lee JA, Lee H, Lee KY, Shin HK. Anti-asthmatic effect of schizandrin on OVA-induced airway inflammation in a murine asthma model. Int Immunopharmacol. 2010; 10(11):1374–1379.

10. Lee NH, Lee MY, Lee JA, Jung DY, Seo CS, Kim JH, Shin HK. Anti-asthmatic effect of Sanguisorba officinalis L. and potential role of heme oxygenase-1 in an ovalbumin-induced murine asthma model. Int J Mol Med. 2010; 26(2):201–208.

11. Lee MY, Seo CS, Lee JA, Lee NH, Kim JH, Ha H, Zheng MS, Son JK, Shin HK. Anti-asthmatic effects of Angelica dahurica against ovalbumin-induced airway inflammation via upregulation of heme oxygenase-1. Food Chem Toxicol. 2011; 49(4):829–837.
12. Sung JE, Lee HA, Kim JE, Yun WB, An BS, Yang SY, Kim DS, Lee CY, Lee HS, Bae CJ, Hwang DY. Saponin-enriched extract of Asparagus cochinchinensis alleviates airway inflammation and remodeling in ovalbumin-induced asthma model. Int J Mol Med. 2017; 40(5):1365–1376.
13. Vadnere GP, Gaud RS, Singhai AK. Evaluation of anti-asthmatic property of Solanum xanthocarpum flower extracts. Pharmacologyonline. 2008; 1:513–522.
14. Tayade PM, Ghaisas MM, Jagtap SA, Dongre SH. Anti-asthmatic activity of methanolic extract of leaves of Tamarindus Indica Linn. J Pharm Res. 2009; 2(5):944–947.
15. Deng YM, Xie QM, Zhang SJ, Yao HY, Zhang H. Anti-asthmatic effects of Perilla seed oil in the guinea pig in vitro and in vivo. Planta Med. 2007; 73(1):53–58.
16. Lee DY, Choo BK, Yoon T, Cheon MS, Lee HW, Lee AY, Kim HK. Anti-inflammatory effects of Asparagus cochinchinensis extract in acute and chronic cutaneous inflammation. J Ethnopharmacol. 2009; 121(1):28–34.
17. Sung JE, Lee HA, Kim JE, Go J, Seo EJ, Yun WB, Kim DS, Son HJ, Lee CY, Lee HS, Hwang DY. Therapeutic effect of ethyl acetate extract from Asparagus cochinchinensis on phthalic anhydride-induced skin inflammation. Lab Anim Res. 2016; 32(1):34–45.
18. Jung KH, Choi HL, Pakr S, Lee G, Kim M, Min JK, Min BI, Bae H. The effects of the standardized herbal formula PM014 on pulmonary inflammation and airway responsiveness in a murine model of cockroach allergen-induced asthma. J Ethnopharmacol. 2014; 155(1):113–122.

19. Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965; 16:144–158.
20. Lee HA, Song BR, Kim HR, Kim JE, Yun WB, Park JJ, Lee ML, Choi JY, Lee HS, Hwang DY. Butanol extracts of Asparagus cochinchinensis fermented with Weissella cibaria inhibit iNOS mediated COX 2 induction pathway and inflammatory cytokines in LPS stimulated RAW264.7 macrophage cells. Exp Ther Med. 2017; 14(5):4986–4994.
21. Jung JY, Lee KY, Lee MY, Jung D, Cho ES, Son HY. Antioxidant and antiasthmatic effects of saucerneol D in a mouse model of airway inflammation. Int Immunopharmacol. 2011; 11(6):698–705.

22. Zhou E, Fu Y, Wei Z, Yu Y, Zhang X, Yang Z. Thymol attenuates allergic airway inflammation in ovalbumin (OVA)-induced mouse asthma. Fitoterapia. 2014; 96:131–137.

23. Mould DR, Green B. Pharmacokinetics and pharmacodynamics of monoclonal antibodies: concepts and lessons for drug development. BioDrugs. 2010; 24(1):23–39.
24. Kim D, Kim SH, Park EJ, Kang CY, Cho SH, Kim S. Anti-allergic effects of PG102, a water-soluble extract prepared from Actinidia arguta, in a murine ovalbumin-induced asthma model. Clin Exp Allergy. 2009; 39(2):280–289.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download