1. Jones CA, Voaklander DC, Johnston DW, Suarez-Almazor ME. Health related quality of life outcomes after total hip and knee arthroplasties in a community based population. J Rheumatol. 2000; 27:1745–1752. PMID:
10914862.
2. Choi HJ, Adiyani L, Sung J, Choi JY, Kim HB, Kim YK, Kwak YG, Yoo H, Lee SO, Han SH, Kim SR, Kim TH, Lee HM, Chun HK, Kim JS, Yoo JD, Koo HS, Cho EH, Lee KW. Korean Nosocomial Infections Surveillance System (KONIS). Five-year decreased incidence of surgical site infections following gastrectomy and prosthetic joint replacement surgery through active surveillance by the Korean Nosocomial Infection Surveillance System. J Hosp Infect. 2016; 93:339–346. PMID:
26944901.

3. Yoon PW, Lee YK, Ahn J, Jang EJ, Kim Y, Kwak HS, Yoon KS, Kim HJ, Yoo JJ. Epidemiology of hip replacements in Korea from 2007 to 2011. J Korean Med Sci. 2014; 29:852–858. PMID:
24932089.

4. Koh IJ, Kim TK, Chang CB, Cho HJ, In Y. Trends in use of total knee arthroplasty in Korea from 2001 to 2010. Clin Orthop Relat Res. 2013; 471:1441–1450. PMID:
23054516.

5. Parvizi J, Fassihi SC, Enayatollahi MA. Diagnosis of periprosthetic joint infection following hip and knee arthroplasty. Orthop Clin North Am. 2016; 47:505–515. PMID:
27241375.

6. Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev. 2014; 27:302–345. PMID:
24696437.

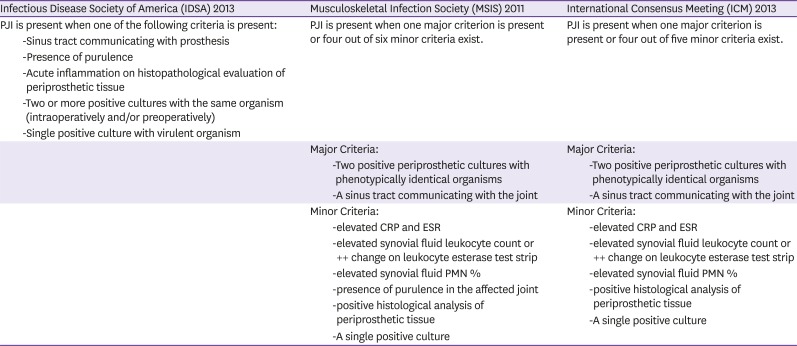
7. Parvizi J, Gehrke T. International Consensus Group on Periprosthetic Joint Infection. Definition of periprosthetic joint infection. J Arthroplasty. 2014; 29:1331. PMID:
24768547.

8. Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011; 469:2992–2994. PMID:
21938532.

9. Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR. Infectious Diseases Society of America. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013; 56:e1–e25. PMID:
23223583.
10. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-defensin accuracy to diagnose periprosthetic joint infection-best available test? J Arthroplasty. 2016; 31:456–460. PMID:
26545577.

11. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014; 472:3254–3262. PMID:
24590839.

12. Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, Shohat N. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018; 33:1309–1314.e2. PMID:
29551303.

13. Koh IJ, Cho WS, Choi NY, Parvizi J, Kim TK. Korea Knee Research Group. How accurate are orthopedic surgeons in diagnosing periprosthetic joint infection after total knee arthroplasty?: a multicenter study. Knee. 2015; 22:180–185. PMID:
25728568.

14. Barrack RL, Harris WH. The value of aspiration of the hip joint before revision total hip arthroplasty. J Bone Joint Surg Am. 1993; 75:66–76. PMID:
8419393.

15. Gomez-Urena EO, Tande AJ, Osmon DR, Berbari EF. Diagnosis of prosthetic joint infection: cultures, biomarker and criteria. Infect Dis Clin North Am. 2017; 31:219–235. PMID:
28483043.
16. McArthur BA, Abdel MP, Taunton MJ, Osmon DR, Hanssen AD. Seronegative infections in hip and knee arthroplasty: periprosthetic infections with normal erythrocyte sedimentation rate and C-reactive protein level. Bone Joint J. 2015; 97-B:939–944. PMID:
26130349.
17. Levine SE, Esterhai JL Jr, Heppenstall RB, Calhoun J, Mader JT. Diagnoses and staging. Osteomyelitis and prosthetic joint infections. Clin Orthop Relat Res. 1993; (295):77–86.

18. Bottner F, Wegner A, Winkelmann W, Becker K, Erren M, Götze C. Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement. J Bone Joint Surg Br. 2007; 89:94–99. PMID:
17259424.
19. Berbari E, Mabry T, Tsaras G, Spangehl M, Erwin PJ, Murad MH, Steckelberg J, Osmon D. Inflammatory blood laboratory levels as markers of prosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am. 2010; 92:2102–2109. PMID:
20810860.
20. Ghanem E, Parvizi J, Burnett RS, Sharkey PF, Keshavarzi N, Aggarwal A, Barrack RL. Cell count and differential of aspirated fluid in the diagnosis of infection at the site of total knee arthroplasty. J Bone Joint Surg Am. 2008; 90:1637–1643. PMID:
18676892.

21. Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med. 2004; 117:556–562. PMID:
15465503.

22. Schinsky MF, Della Valle CJ, Sporer SM, Paprosky WG. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J Bone Joint Surg Am. 2008; 90:1869–1875. PMID:
18762646.

23. Cipriano CA, Brown NM, Michael AM, Moric M, Sporer SM, Della Valle CJ. Serum and synovial fluid analysis for diagnosing chronic periprosthetic infection in patients with inflammatory arthritis. J Bone Joint Surg Am. 2012; 94:594–600. PMID:
22488615.

24. Lee YS, Koo KH, Kim HJ, Tian S, Kim TY, Maltenfort MG, Chen AF. Synovial fluid biomarkers for the diagnosis of periprosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am. 2017; 99:2077–2084. PMID:
29257013.
25. Frangiamore SJ, Saleh A, Kovac MF, Grosso MJ, Zhang X, Bauer TW, Daly TM, Ricchetti ET, Iannotti JP. Synovial fluid interleukin-6 as a predictor of periprosthetic shoulder infection. J Bone Joint Surg Am. 2015; 97:63–70. PMID:
25568396.

26. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-Defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014; 96:1439–1445. PMID:
25187582.

27. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014; 472:4006–4009. PMID:
25256621.

28. Frangiamore SJ, Saleh A, Grosso MJ, Kovac MF, Higuera CA, Iannotti JP, Ricchetti ET. α-Defensin as a predictor of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2015; 24:1021–1027. PMID:
25672257.

29. Plate A, Stadler L, Sutter R, Anagnostopoulos A, Frustaci D, Zbinden R, Fucentese SF, Zinkernagel AS, Zingg PO, Achermann Y. Inflammatory disorders mimicking periprosthetic joint infections may result in false positive α-defensin. Clin Microbiol Infect. 2018; pii:S1198-743X(18)30194-0.
30. Tsaras G, Maduka-Ezeh A, Inwards CY, Mabry T, Erwin PJ, Murad MH, Montori VM, West CP, Osmon DR, Berbari EF. Utility of intraoperative frozen section histopathology in the diagnosis of periprosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am. 2012; 94:1700–1711. PMID:
22992880.
31. Shah RP, Plummer DR, Moric M, Sporer SM, Levine BR, Della Valle CJ. Diagnosing infection in the setting of periprosthetic fractures. J Arthroplasty. 2016; 31(9 Suppl):140–143. PMID:
27067465.

32. Bori G, Soriano A, García S, Gallart X, Mallofre C, Mensa J. Neutrophils in frozen section and type of microorganism isolated at the time of resection arthroplasty for the treatment of infection. Arch Orthop Trauma Surg. 2009; 129:591–595. PMID:
18600336.

33. Hughes JG, Vetter EA, Patel R, Schleck CD, Harmsen S, Turgeant LT, Cockerill FR 3rd. Culture with BACTEC Peds Plus/F bottle compared with conventional methods for detection of bacteria in synovial fluid. J Clin Microbiol. 2001; 39:4468–4471. PMID:
11724863.

34. Font-Vizcarra L, García S, Martínez-Pastor JC, Sierra JM, Soriano A. Blood culture flasks for culturing synovial fluid in prosthetic joint infections. Clin Orthop Relat Res. 2010; 468:2238–2243. PMID:
20162386.

35. Gandhi R, Silverman E, Courtney PM, Lee GC. How many cultures are necessary to identify pathogens in the management of total hip and knee arthroplasty infections? J Arthroplasty. 2017; 32:2825–2828. PMID:
28479058.

36. Larsen LH, Lange J, Xu Y, Schønheyder HC. Optimizing culture methods for diagnosis of prosthetic joint infections: a summary of modifications and improvements reported since 1995. J Med Microbiol. 2012; 61:309–316. PMID:
22222201.

37. Hughes HC, Newnham R, Athanasou N, Atkins BL, Bejon P, Bowler IC. Microbiological diagnosis of prosthetic joint infections: a prospective evaluation of four bacterial culture media in the routine laboratory. Clin Microbiol Infect. 2011; 17:1528–1530. PMID:
21851478.

38. Peel TN, Dylla BL, Hughes JG, Lynch DT, Greenwood-Quaintance KE, Cheng AC, Mandrekar JN, Patel R. Improved diagnosis of prosthetic joint infection by culturing periprosthetic tissue specimens in blood culture bottles. MBio. 2016; 7:e01776–15. PMID:
26733067.

39. Yan Q, Karau MJ, Greenwood-Quaintance KE, Mandrekar JN, Osmon DR, Abdel MP, Patel R. Comparison of diagnostic accuracy of periprosthetic tissue culture in blood culture bottles to that of prosthesis sonication fluid culture for diagnosis of prosthetic joint infection (PJI) by use of bayesian latent class modeling and IDSA PJI criteria for classification. J Clin Microbiol. 2018; 56:pii:e00319–18.

40. Peel TN, Sedarski JA, Dylla BL, Shannon SK, Amirahmadi F, Hughes JG, Cheng AC, Patel R. Laboratory workflow analysis of culture of periprosthetic tissues in blood culture bottles. J Clin Microbiol. 2017; 55:2817–2826. PMID:
28701418.

41. Peel TN, Spelman T, Dylla BL, Hughes JG, Greenwood-Quaintance KE, Cheng AC, Mandrekar JN, Patel R. Optimal periprosthetic tissue specimen number for diagnosis of prosthetic joint infection. J Clin Microbiol. 2016; 55:234–243. PMID:
27807152.

42. Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis. 2008; 47:1403–1409. PMID:
18937579.

43. Butler-Wu SM, Burns EM, Pottinger PS, Magaret AS, Rakeman JL, Matsen FA 3rd, Cookson BT. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011; 49:2490–2495. PMID:
21543562.

44. Trampuz A, Piper KE, Hanssen AD, Osmon DR, Cockerill FR, Steckelberg JM, Patel R. Sonication of explanted prosthetic components in bags for diagnosis of prosthetic joint infection is associated with risk of contamination. J Clin Microbiol. 2006; 44:628–631. PMID:
16455930.

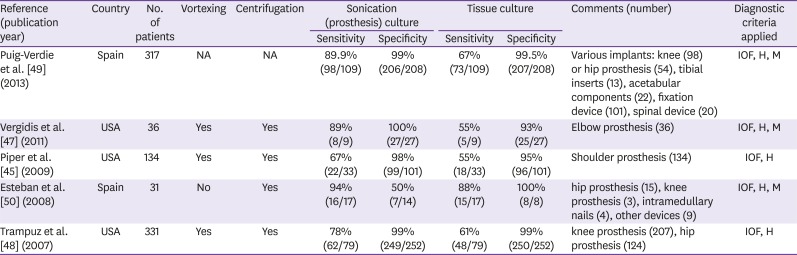
45. Piper KE, Jacobson MJ, Cofield RH, Sperling JW, Sanchez-Sotelo J, Osmon DR, McDowell A, Patrick S, Steckelberg JM, Mandrekar JN, Fernandez Sampedro M, Patel R. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J Clin Microbiol. 2009; 47:1878–1884. PMID:
19261785.

46. Cazanave C, Greenwood-Quaintance KE, Hanssen AD, Karau MJ, Schmidt SM, Gomez Urena EO, Mandrekar JN, Osmon DR, Lough LE, Pritt BS, Steckelberg JM, Patel R. Rapid molecular microbiologic diagnosis of prosthetic joint infection. J Clin Microbiol. 2013; 51:2280–2287. PMID:
23658273.

47. Vergidis P, Greenwood-Quaintance KE, Sanchez-Sotelo J, Morrey BF, Steinmann SP, Karau MJ, Osmon DR, Mandrekar JN, Steckelberg JM, Patel R. Implant sonication for the diagnosis of prosthetic elbow infection. J Shoulder Elbow Surg. 2011; 20:1275–1281. PMID:
22078323.

48. Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007; 357:654–663. PMID:
17699815.

49. Puig-Verdié L, Alentorn-Geli E, González-Cuevas A, Sorlí L, Salvadó M, Alier A, Pelfort X, Portillo ME, Horcajada JP. Implant sonication increases the diagnostic accuracy of infection in patients with delayed, but not early, orthopaedic implant failure. Bone Joint J. 2013; 95-B:244–249. PMID:
23365036.

50. Esteban J, Gomez-Barrena E, Cordero J, Martín-de-Hijas NZ, Kinnari TJ, Fernandez-Roblas R. Evaluation of quantitative analysis of cultures from sonicated retrieved orthopedic implants in diagnosis of orthopedic infection. J Clin Microbiol. 2008; 46:488–492. PMID:
18077647.

51. Liu H, Zhang Y, Li L, Zou HC. The application of sonication in diagnosis of periprosthetic joint infection. Eur J Clin Microbiol Infect Dis. 2017; 36:1–9. PMID:
27649698.

52. Zhai Z, Li H, Qin A, Liu G, Liu X, Wu C, Li H, Zhu Z, Qu X, Dai K. Meta-analysis of sonication fluid samples from prosthetic components for diagnosis of infection after total joint arthroplasty. J Clin Microbiol. 2014; 52:1730–1736. PMID:
24574298.

53. Wadey VM, Huddleston JI, Goodman SB, Schurman DJ, Maloney WJ, Baron EJ. Use and cost-effectiveness of intraoperative acid-fast bacilli and fungal cultures in assessing infection of joint arthroplasties. J Arthroplasty. 2010; 25:1231–1234. PMID:
19879728.

54. Million M, Bellevegue L, Labussiere AS, Dekel M, Ferry T, Deroche P, Socolovschi C, Cammilleri S, Raoult D. Culture-negative prosthetic joint arthritis related to Coxiella burnetii. Am J Med. 2014; 127:786.e7–e10.
55. Kim HJ. Current status of tuberculosis in Korea. Korean J Med. 2011; 82:257–262.

56. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014; 69(Suppl 1):i21–4. PMID:
25135084.

57. Jun Y, Jianghua L. Diagnosis of periprosthetic joint infection using polymerase chain reaction: an updated systematic review and meta-analysis. Surg Infect (Larchmt). 2018; 19:555–565. PMID:
29920159.

58. Melendez DP, Greenwood-Quaintance KE, Berbari EF, Osmon DR, Mandrekar JN, Hanssen AD, Patel R. Evaluation of a genus- and group-specific rapid PCR assay panel on synovial fluid for diagnosis of prosthetic knee infection. J Clin Microbiol. 2016; 54:120–126. PMID:
26537446.

59. Suda AJ, Tinelli M, Beisemann ND, Weil Y, Khoury A, Bischel OE. Diagnosis of periprosthetic joint infection using alpha-defensin test or multiplex-PCR: ideal diagnostic test still not found. Int Orthop. 2017; 41:1307–1313. PMID:
28160020.

60. Lourtet-Hascoëtt J, Bicart-See A, Félicé MP, Giordano G, Bonnet E. Is Xpert MRSA/SA SSTI real-time PCR a reliable tool for fast detection of methicillin-resistant coagulase-negative staphylococci in periprosthetic joint infections? Diagn Microbiol Infect Dis. 2015; 83:59–62. PMID:
26052062.

61. Ryu SY, Greenwood-Quaintance KE, Hanssen AD, Mandrekar JN, Patel R. Low sensitivity of periprosthetic tissue PCR for prosthetic knee infection diagnosis. Diagn Microbiol Infect Dis. 2014; 79:448–453. PMID:
24972853.

62. Renz N, Feihl S, Cabric S, Trampuz A. Performance of automated multiplex PCR using sonication fluid for diagnosis of periprosthetic joint infection: a prospective cohort. Infection. 2017; 45:877–884. PMID:
28983865.

63. Gomez E, Cazanave C, Cunningham SA, Greenwood-Quaintance KE, Steckelberg JM, Uhl JR, Hanssen AD, Karau MJ, Schmidt SM, Osmon DR, Berbari EF, Mandrekar J, Patel R. Prosthetic joint infection diagnosis using broad-range PCR of biofilms dislodged from knee and hip arthroplasty surfaces using sonication. J Clin Microbiol. 2012; 50:3501–3508. PMID:
22895042.

64. Thoendel M, Jeraldo P, Greenwood-Quaintance KE, Yao J, Chia N, Hanssen AD, Abdel MP, Patel R. Identification of prosthetic joint infection pathogens using a shotgun metagenomics approach. Clin Infect Dis. 2018; [Epub ahead of print].

65. Tarabichi M, Shohat N, Goswami K, Alvand A, Silibovsky R, Belden K, Parvizi J. Diagnosis of periprosthetic joint infection: the potential of next-generation sequencing. J Bone Joint Surg Am. 2018; 100:147–154. PMID:
29342065.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download