Abstract
The present study evaluated the survival impact of standard adjuvant chemotherapy and prognostic differences between Epstein-Barr virus (EBV)-associated gastric cancer (EBVaGC) and EBV-negative gastric cancer (EBVnGC). A total of 276 patients were enrolled according to the following criteria: 1) pathologically diagnosed with primary gastric adenocarcinoma, 2) test results from EBV-encoded RNA in situ hybridization, 3) stage II/III according to the 7th edition of UICC/AJCC staging system for gastric cancer, and 4) postoperative adjuvant chemotherapy. Fifty-nine (21.4%) and 217 (78.6%) patients exhibited EBVaGC and EBVnGC, respectively, while 129 (46.7%) patients were classified as stage II and 147 (53.3%) as stage III. As for adjuvant chemotherapy, 87 (31.5%) patients received capecitabine and oxaliplatin, while 189 (68.5%) received S-1 monotherapy. With a median follow-up duration of 21.3 (6.4-89.0) months, the estimated 3-year disease-free survival (DFS) and overall survival (OS) rates were 74.8% and 83.0%, respectively. In univariate analysis and multivariate analysis using a Cox proportional hazard model including age, gender, stage, Lauren classification, and the type of chemotherapy, EBV-positivity was not significantly associated with DFS (p-value= 0.630) regardless of the type of chemotherapy. Therefore, no association was found between EBV positivity and the survival outcomes in patients with curatively resected gastric cancer who received standard adjuvant chemotherapy.
Go to : 
Comprehensive molecular characterization of gastric cancer (GC) has identified Epstein-Barr virus (EBV)-associated gastric cancer (EBVaGC) as one of four GC subtypes.1 Representing nearly 10% of all GC worldwide, EBVaGC is defined by the presence of EBV in the GC cells.2 EBV infection contributes to the malignant transformation of GC cells through disrupting various cellular processes and signaling pathways.34 Recent studies have also differentiated EBVaGC with unique genomic aberrations and clinicopathologic features, including less lymph node involvement and a better prognosis.56 However, it is still unknown if the EBV subtype can also be associated with different clinical benefits from adjuvant chemotherapy.
Several studies have already demonstrated an improved survival with adjuvant chemotherapy following surgery with D2 lymph node dissection for GC.789 Thus, many countries have accepted oxaliplatin plus capecitabine (XELOX) combination chemotherapy or S-1 monotherapy as the standard adjuvant chemotherapy for GC.710 Notwithstanding, 30–50% of patients still experience tumor recurrence following adjuvant chemotherapy and the clinical relevance of the four molecular subtypes, such as the implications for prognosis and response to standard adjuvant chemotherapy have not yet been established.1112 Moreover, the heterogeneity of GC is recognized as one of the major difficulties when choosing a treatment approach and determining the reasons for worse treatment outcomes.131415 However, no published study has yet investigated the predictive value of EBV-positivity with resected GC followed by adjuvant chemotherapy. Therefore, the purpose of this study was to analyze the survival differences according to the EBV-positivity in GC.
Go to : 
This study retrospectively reviewed 773 patients with GC who underwent surgical resection at Kyungpook National University Chilgok Hospital (KNUCH) between January 2011 and December 2017. The patients were enrolled according to the following criteria: 1) pathologically diagnosed with primary gastric adenocarcinoma, 2) test results for EBV-encoded RNA in situ hybridization, 3) stage II/III according to the 7th edition of Union for International Cancer Control/American Joint Committee on Cancer (UICC/AJCC) staging system for GC,16 and 4) postoperative adjuvant chemotherapy. A total of 276 patients met these criteria and were included in this study. The patient records were also reviewed for data on their medical history, age, sex, backbone chemotherapy regimen, surgical methods, and pathologic results. This study was approved by the institutional review board at KNUCH (KNUCH 2016-05-012-002).
The adjuvant chemotherapy treatment was begun 3 to 6 weeks after surgery. In the case of S-1 monotherapy, the patients received S-1 orally twice daily on days 1 to 28, followed by a 14-day recovery period, which was repeated for 1 year. In the case of XELOX, the patients received intravenous oxaliplatin in day 1 and oral capecitabine twice a day on days 1 to 14, followed by a 7-day recovery period, which was repeated for 6 months.
The descriptive statistics are reported as proportions and medians. The categorical variables were evaluated using a Chi-square test and Fisher's exact test, as appropriate. Overall survival (OS) was defined as the interval between the time of surgery and death, or else censored at the date of last contact in medical records. Disease-free survival (DFS) was defined as the interval between the time of surgery and evidence of disease recurrence, or else censored at the date of death or last contact. The Kaplan-Meier method was used to estimate the OS and DFS. The survival curves were compared using a log rank test according to the EBV expression differences. Multivariate survival analyses were carried out using the Cox proportional hazard regression model. The hazard ratio (HR) and 95% confidence interval (CI) were evaluated for each factor. A p-value <0.05 was considered statistically significant. The statistical analyses were performed using SPSS for Windows (version 19.0, SPSS Inc., Chicago, Ill., USA).
Go to : 
The patient characteristics are summarized in Table 1. The median age was 63.5 years (range 35–84 years) and 201 (72.6%) patients were male. Most of the tumors were located in the body (43.3%) and antrum (42.6%) of the stomach. While 107 (38.6%) patients underwent a total gastrectomy, 167 (60.3%) received a subtotal gastrectomy. After surgical resection, 129 (46.7%) patients were classified as stage II and 147 (53.3%) as stage III. The major histology was poorly differentiated adenocarcinoma (39.7%). According to EBV-encoded RNA in situ hybridization, 59 (21.4%) and 217 (78.6%) patients exhibited EBVaGC and EBVnGC, respectively. Among the 276 eligible patients, 87 patients (31.5%) received XELOX, and the other 189 (68.5%) received S-1 as their adjuvant chemotherapy.
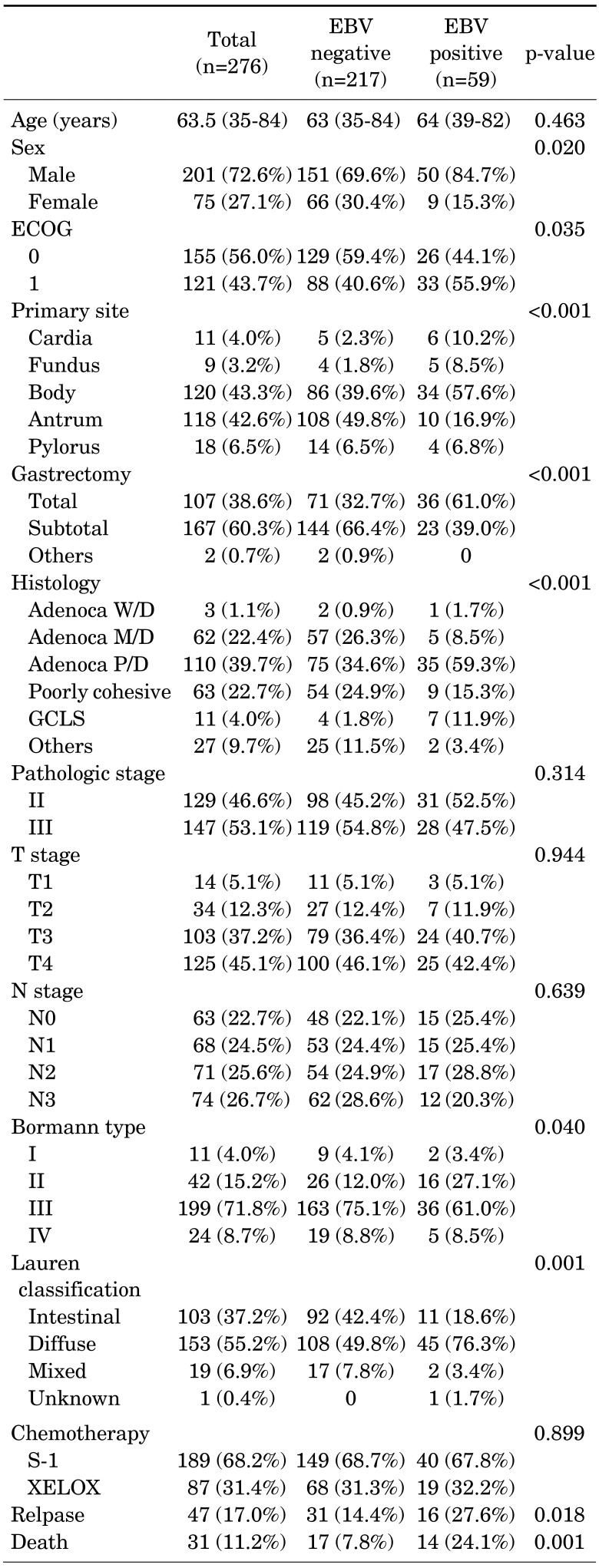
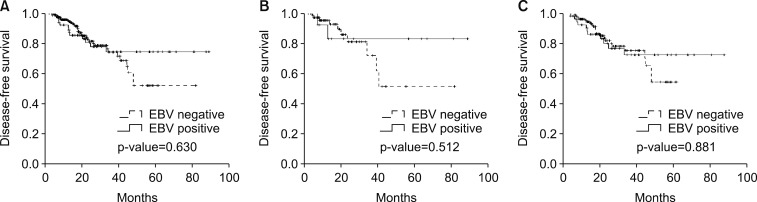
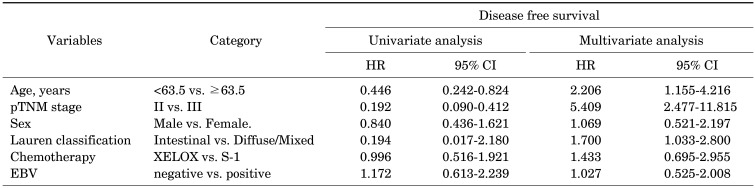
With a median follow-up duration of 21.3 months (6.4–89.0), the estimated 3-year DFS and OS rates were 74.8% and 83.0%, respectively. During the analyses, 47 (17%) patients experienced recurrence and 31 (11.2%) patients died. While the survival outcomes for EBVaGC showed a better tendency towards DFS, there were no statistically significant differences (p-value=0.630; Fig. 1A). In the subgroup analysis according to the type of chemotherapy, EBV-positivity did not have a significant effect on DFS (Fig. 1B, C). In the multivariate analysis adjusted for age, gender, stage, Lauren classification, type of chemotherapy, and EBV-positivity, no significant association was found between EBV-positivity and DFS (p-value= 0.831; Table 2), while the stage and Lauren classification were identified as independent prognostic factors for DFS.
Go to : 
This study investigated whether the clinical benefit of EBV-positivity differed in the case of GC. To the best of our knowledge, this is the first study to evaluate the predictive role of the EBV status in resected GC followed by adjuvant chemotherapy. However, for the patients in the current series EBV-positivity did not demonstrate a predictive value in a multivariate analysis adjusted for the tumor stage and type of chemotherapy.
EBV positive tumors display extreme DNA hypermethylation, phosphatidylinositol 3-kinase catalytic subunit (PIK3CA) mutation, and recurrent Janus kinase 2 (JAK2) amplifications.117 They also exhibit interleukin-12 mediated signaling activation and programmed death-ligand 1 (PD-L1)/-ligand 2 (PD-L2) overexpression compared to other cancers, as reported by The Cancer Genome Atlas (TCGA).118 Therefore, this heterogeneity with regard to molecular alterations may vary the response to standard chemotherapy. While the EBV status showed no significant effect on the survival outcomes for the present series of resected GC followed by adjuvant chemotherapy, several studies have reported on the clinical significance of molecular biology in GC. Sohn et al.11 analyzed the subtype-specific benefit of adjuvant chemotherapy using genetic signatures to validate TCGA data. They found that patients with the chromosomal instability (CIN) subtype benefited the most from adjuvant chemotherapy, while patients with the microsatellite instability (MSI) and genomically stable (GS) subtype showed a better tendency, yet with not significantly. Similarly, the results from the adjuvant capecitabine plus oxaliplatin for GC after D2 gastrectomy trial found no significant benefit of capecitabine plus oxaliplatin-based adjuvant chemotherapy in patients with a high level of MSI (MSI-H) GC.19 However, the efficacy of adjuvant chemotherapy has not been assessed in EBV subtype patients. Interestingly, recent preclinical studies reported that EBV infection contributes to the chemo-resistance of 5-fluorouracil (5FU) and docetaxel in GC by modulating apoptosis related cellular genes.2021 As these findings point to the possibility that adjuvant chemotherapy including 5FU and oxaliplatin affect the survival outcomes in EBVaGC, the current study results indicate that the role of adjuvant chemotherapy in EBVaGC still requires further clarification.
While the present study found that EBV-positivity has no predictive value of adjuvant chemotherapy, several recent studies have also reported on the prognostic significance of the EBV status in GC. Song et al.22 noted a significantly longer OS and DFS for patients with lymphoepithelioma-like carcinoma subtype of EBVaGC, suggesting that the prognosis of EBVaGC may depend on the patient's inflammatory response. However, another study found no survival differences between EBVaGC and EBVnGC in a study that retrospectively evaluated 1,020 patients with stage I-III GC who underwent a radical gastrectomy and lymphadenectomy.23 The latter findings are consistent with the present study in terms of the stage at the time of surgery and treatment applications. Thus, the precise relationship between EBV infection and the prognosis for GC still needs to be clarified, along with the inconsistent results in previous studies. Although the current study found no survival outcome association, EBV infection is well known to be related with tumor progression and metastasis in GC.2425 In particular, biological characteristics of stage IV GC may differ from those of early stage GC.26 Therefore, the predictive role of chemotherapy for stage IV GC should be evaluated in further studies aimed at elucidating the clinical significance of each chemotherapy.
While the present data did not indicate a significant predictive role for EBV-positivity in the case of adjuvant chemotherapy, the results should be cautiously evaluated due to certain limitations. First, the current study is a retrospective evaluation. Second, EBVnGC included the CIN, GS, and MSI subtypes of GC and the response to chemotherapy of the four GC subtypes may vary according to their fundamental nature. Plus, genetic mutations can also affect the outcome of chemotherapy in cancer. Moreover, no detailed information was provided on the cycles and number of chemotherapies and treatments after recurrence. Lastly, the sample size was small to compare between two groups, and follow-up duration was relatively short.
In conclusion, no association was found between EBV-positivity and the survival outcomes in patients with curatively resected gastric cancer who received standard adjuvant chemotherapy.
Go to : 
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (2014R1A5A2009242) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1D1A3B03032320).
Go to : 
References
1. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014; 513:202–209. PMID: 25079317.
2. Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009; 137:824–833. PMID: 19445939.
3. zur Hausen A, van Grieken NC, Meijer GA, Hermsen MA, Bloemena E, Meuwissen SG, et al. Distinct chromosomal aberrations in Epstein-Barr virus-carrying gastric carcinomas tested by comparative genomic hybridization. Gastroenterology. 2001; 121:612–618. PMID: 11522745.
4. Wu MS, Shun CT, Wu CC, Hsu TY, Lin MT, Chang MC, et al. Epstein-Barr virus-associated gastric carcinomas: relation to H. pylori infection and genetic alterations. Gastroenterology. 2000; 118:1031–1038. PMID: 10833477.
5. van Beek J, zur Hausen A, Klein Kranenbarg E, van de Velde CJ, Middeldorp JM, van den Brule AJ, et al. EBV-positive gastric adenocarcinomas: a distinct clinicopathologic entity with a low frequency of lymph node involvement. J Clin Oncol. 2004; 22:664–670. PMID: 14966089.
6. Shinozaki-Ushiku A, Kunita A, Fukayama M. Update on Epstein-Barr virus and gastric cancer (review). Int J Oncol. 2015; 46:1421–1434. PMID: 25633561.
7. Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014; 15:1389–1396. PMID: 25439693.
8. GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group. Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010; 303:1729–1737. PMID: 20442389.
9. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007; 357:1810–1820. PMID: 17978289.
10. Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011; 29:4387–4393. PMID: 22010012.
11. Sohn BH, Hwang JE, Jang HJ, Lee HS, Oh SC, Shim JJ, et al. Clinical significance of four molecular subtypes of gastric cancer identified by the cancer genome atlas project. Clin Cancer Res. 2017; [Epub ahead of print]. DOI: 10.1158/1078-0432.CCR-16-2211.
12. Aoyama T, Yoshikawa T, Watanabe T, Hayashi T, Ogata T, Cho H, et al. Survival and prognosticators of gastric cancer that recurs after adjuvant chemotherapy with S-1. Gastric Cancer. 2011; 14:150–154. PMID: 21327443.
13. Wang K, Kan J, Yuen ST, Shi ST, Chu KM, Law S, et al. Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nat Genet. 2011; 43:1219–1223. PMID: 22037554.
14. Zang ZJ, Cutcutache I, Poon SL, Zhang SL, McPherson JR, Tao J, et al. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nat Genet. 2012; 44:570–574. PMID: 22484628.
15. Zouridis H, Deng N, Ivanova T, Zhu Y, Wong B, Huang D, et al. Methylation subtypes and large-scale epigenetic alterations in gastric cancer. Sci Transl Med. 2012; 4:156ra140.
16. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–1474. PMID: 20180029.
17. Sukawa Y, Yamamoto H, Nosho K, Kunimoto H, Suzuki H, Adachi Y, et al. Alterations in the human epidermal growth factor receptor 2-phosphatidylinositol 3-kinase-v-Akt pathway in gastric cancer. World J Gastroenterol. 2012; 18:6577–6586. PMID: 23236232.
18. Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015; 21:449–456. PMID: 25894828.
19. Choi YY, Kim H, Shin SJ, Kim HY, Lee J, Yang HK, et al. Microsatellite instability and programmed cell death-ligand 1 expression in stage II/III gastric cancer: post hoc analysis of the CLASSIC randomized controlled study. Ann Surg. 2018; [Epub ahead of print]. DOI: 10.1097/SLA.0000000000002803.
20. Seo JS, Kim TG, Hong YS, Chen JY, Lee SK. Contribution of Epstein-Barr virus infection to chemoresistance of gastric carcinoma cells to 5-fluorouracil. Arch Pharm Res. 2011; 34:635–643. PMID: 21544729.
21. Shin HJ, Kim DN, Lee SK. Association between Epstein-Barr virus infection and chemoresistance to docetaxel in gastric carcinoma. Mol Cells. 2011; 32:173–179. PMID: 21626300.
22. Song HJ, Srivastava A, Lee J, Kim YS, Kim KM, Ki Kang W, et al. Host inflammatory response predicts survival of patients with Epstein-Barr virus-associated gastric carcinoma. Gastroenterology. 2010; 139:84–92. PMID: 20398662.
23. Huang SC, Ng KF, Chen KH, Hsu JT, Liu KH, Yeh TS, et al. Prognostic factors in Epstein-Barr virus-associated stage I-III gastric carcinoma: implications for a unique type of carcinogenesis. Oncol Rep. 2014; 32:530–538. PMID: 24899228.
24. Naseem M, Barzi A, Brezden-Masley C, Puccini A, Berger MD, Tokunaga R, et al. Outlooks on Epstein-Barr virus associated gastric cancer. Cancer Treat Rev. 2018; 66:15–22. PMID: 29631196.
25. Huang SCM, Tsao SW, Tsang CM. Interplay of viral infection, host cell factors and tumor microenvironment in the pathogenesis of nasopharyngeal carcinoma. Cancers (Basel). 2018; 10:E106. PMID: 29617291.
26. Fu DG. Epigenetic alterations in gastric cancer (review). Mol Med Rep. 2015; 12:3223–3230. PMID: 25997695.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download