This article has been
cited by other articles in ScienceCentral.
Abstract
Neuroendocrine tumors (NETs) arising from the pancreas and duodenum together represent 18% of all gastroenteropancreatic NETs. Duodenal neuroendocrine tumor (D-NET) comprises 1%–3% of all primary duodenal tumors, of these only 20% are resectable. Surgery remains the only curative option provided a R0 resection can be done. We present a case of D-NET in an elderly male who was being investigated for anemia who was treated with pancreaticoduodenectomy and was discharged without sequele.
Keywords: Duodenal neuroendocrine tumor, WHO classification, Pancreaticoduodenectomy, Anemia
INTRODUCTION
Duodenal neuroendocrine tumors (D-NETs) are rare comprising 1%–3% of all primary duodenal tumors, of these only 20% are resectable (
1). World Health Organization classification (2010) proposed a grading system for neuroendocrine tumors (NETs) based on the proliferative activity of tumor cells (the number of mitoses or the Ki-67 labeling index). NETs have been classified as G1 (low grade), G2 (intermediate grade), and G3 (neuroendocrine carcinoma). It has prognostic significance and is independent of tumor stage.
The 90% of the D-NETs are non-functional (
2). Non-functioning NET can be detected by serum chromogranin A (CgA) levels. A triple-phase contrast-enhanced helical computed tomography (CT) or a magnetic resonance imaging (MRI) scan is usually done to rule out liver metastases and for staging. Surgery remains the only curative option provided a R0 resection can be achieved.
CASE REPORT
A 58 years old male presented with abdominal pain, anemia and weakness since 2 months. Clinical examination was normal. On diagnostic upper gastrointestinal (UGI) endoscopy there were multiple submucosal nodules on the anterior wall of the duodenal bulb, located at the junction of D1 and D2. Biopsy revealed grade 1 D-NET. There was no evidence of liver metastasis on contrast enhanced CT (
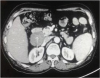
Fig. 1). Positron emission computed tomography (PET-CT) scan showed a heterogeneously enhancing mass lesion of size 4.2×4.7×6.1 cm at the duodenal bulb extending into D2 with low fluorodeoxyglucose (FDG) avidity.
Fig. 1
CT scan showing nodal lesion at the junction of D1 and D2 (white arrow).
CT = computed tomography.
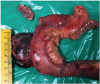
Intraoperatively, 6×4 cm firm mass was seen arising from the posterolateral aspect of the second part of duodenum. Surrounding tissue planes were maintained. Pancreaticoduodenectomy with enemas removal of loco-regional lymph nodes was performed (
Fig. 2). On histopathology examination the tumor was diagnosed as neuroendocrine tumor situated in submucosa and superficial muscularis propria of the duodenum (grade 1). Tumor was positive for synaptophysin and CgA. Ki-67 labelling index was 1%. Anterior and posterior pancreaticoduodenal lymph nodes were positive for metastasis with perinodal extension. The patient was started on jejunostomy feeds by day 3 and on orals by day 5. He was discharged on day 8. At one year follow up there is no evidence of recurrence of tumor.
Fig. 2
Operative specimen of the pancreaticoduodenectomy.
DISCUSSION
D-NETs comprise 1%–3% of primary duodenal tumors, 5%–8% of all gastroenteropancreatic neuroendocrine tumors (G-NETs) (
3). They are diagnosed more frequently now due to high-resolution imaging and endoscopy. The ileum remains the most frequent site of NETs in the small intestine (>70%) (
4).
Most D-NETs are detected incidentally during UGI endoscopy for other symptoms. The most common symptoms that lead to their detection are vague abdominal pain (37%), UGI bleeding (21%), anemia (21%), and jaundice (18%) (
2). Serum gastrin and serum CgA levels should be determined in patients with D-NETs. Our patient presented with anemia, the cause of which was diagnosed as D-NET on endoscopic biopsy.
The algorithm of diagnostic imaging starts with endoscopy and a tissue sample for histological diagnosis (
5). CT scanning of the abdomen and thorax as well as somatostatin receptor scintigraphy (SRS) are the major imaging modalities used to establish tumor staging and localization. 68-Gallium-DOTATOC-PET/CT is used for localizing NETs in patients where there is a high index of suspicion and all previous imaging have failed to localize it (
6).
Endosonography is done to evaluate the size and depth of infiltration of the tumor, layer of origin, the loco-regional lymph node involvement and feasibility of endoscopic resection (
7).
The European Neuroendocrine Tumor Society recommends endoscopic treatment for G-NETs ≤10 mm that do not extend beyond the submucosa and do not demonstrate lymph node involvement or distant metastasis. D-NETs of >2 cm or of any size with lymph node involvement to be treated by surgical resection (
8). Our patient had non-functioning D-NET with regional lymph node involvement for which he underwent pancreaticoduodenectomy with R0 resection.
Peptide receptor chemo radionuclide therapy has been shown to be effective in inoperable D-NET and also has a role in neoadjuvant therapy for unresectable metastatic disease with 15% to 35% response rate (
9). Surgical resection of D-NETs should be followed up by multislice CT, SRS and CgA levels performed at 6 and 12 months after surgery, and then annually for a minimum of 3 years (
8).
In conclusion, non-functioning NET's are difficult to diagnose at the early stage. Therefore, there is a need for UGI scopy in patients with vague abdominal pain, UGI bleeding and anemia. Surgery is only curative modality in the early stage. This case demonstrates the importance of clinical correlation of symptoms and laboratory investigations to guide appropriate imaging modalities to identify the cause. Clinician must keep a differential diagnosis of a NET in mind when dealing with a case of duodenal mass in order to provide a definitive treatment.
ACKNOWLEDGEMENTS
We wish to thank Dr. A. N. Supe, director, (ME & MH) & Dean, Seth G.S. Medical College and K.E.M. Hospital, for permitting us to publish hospital data.
References
1. Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008; 26:3063–3072.

2. Hoffmann KM, Furukawa M, Jensen RT. Duodenal neuroendocrine tumors: Classification, functional syndromes, diagnosis and medical treatment. Best Pract Res Clin Gastroenterol. 2005; 19:675–697.

3. Modlin IM, Champaneria MC, Chan AK, Kidd M. A three-decade analysis of 3,911 small intestinal neuroendocrine tumors: the rapid pace of no progress. Am J Gastroenterol. 2007; 102:1464–1473.

4. Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009; 249:63–71.
5. Eriksson B, Klöppel G, Krenning E, Ahlman H, Plöckinger U, Wiedenmann B, et al. Consensus guidelines for the management of patients with digestive neuroendocrine tumors--well-differentiated jejunal-ileal tumor/carcinoma. Neuroendocrinology. 2008; 87:8–19.

6. Schraml C, Schwenzer NF, Sperling O, Aschoff P, Lichy MP, Müller M, et al. Staging of neuroendocrine tumours: comparison of [⁶⁸Ga]DOTATOC multiphase PET/CT and whole-body MRI. Cancer Imaging. 2013; 13:63–72.

7. Kim MK. Endoscopic ultrasound in gastroenteropancreatic neuroendocrine tumors. Gut Liver. 2012; 6:405–410.

8. Delle Fave G, O'Toole D, Sundin A, Taal B, Ferolla P, Ramage JK, et al. ENETS consensus guidelines update for gastroduodenal neuroendocrine neoplasms. Neuroendocrinology. 2016; 103:119–124.

9. Barber TW, Hofman MS, Thomson BN, Hicks RJ. The potential for induction peptide receptor chemoradionuclide therapy to render inoperable pancreatic and duodenal neuroendocrine tumours resectable. Eur J Surg Oncol. 2012; 38:64–71.



