Abstract
Primary sarcomas of the liver are unusual neoplasms developing in adults. They constitute a heterogeneous group of neoplasms including undifferentiated embryonal sarcoma. Patients usually present with an abdominal mass and abdominal pain. Case 1: A 53-year-old woman presented with abdominal pain. Computed tomography showed an occupying mass in the right lobule and an intra-auricular multi-lobulated mass suggestive of a secondary deposit. Biopsy of the hepatic lesion revealed undifferentiated embryonal sarcoma. Despite radiotherapy and supportive measures, her overall status progressively worsened until cardiac arrest. Case 2: A 41-year-old woman presented with hepatomegaly. Abdominal imaging showed cystic lesions in the right hepatic lobule with multiple septae. The patient underwent extended right hepatectomy and a histopathological study reported high-grade undifferentiated embryonal sarcoma. Two years after surgery, a new cystic lesion in the surgical site was recorded and chemotherapy was scheduled. The lesion remained stable for three years when disease progression was observed and second-line chemotherapy was initiated. Although undifferentiated embryonal sarcoma of the liver has poor prognosis, early diagnosis is essential to increase the chances of survival. Currently, surgical resection and chemotherapy are the primary treatment modalities.
Primary liver sarcomas are unusual neoplasms in adulthood and constitute approximately 0.2% of all primary liver tumors.1 They represent a heterogeneous group of neoplasms including undifferentiated embryonal sarcoma (UESL), which was first described by Stocker and Ishak2 in 1978 as a mesenchymal tumor without any specific differentiation. Until now, few adult patients with UESL have been reported in the literature, with a slight preference for females and a mean age at diagnosis of 51.3 Although UESL is associated with prognosis with a reported survival time of less than one year, early diagnosis is essential to increase the overall survival.4
A 53-year-old female presented with abdominal distension and pain localized to the right upper abdominal quadrant radiating to the back. It was partially controlled with oral analgesia and was associated with fever. Due to pain progression, the patient was treated at the emergency department three months later for diaphoresis and dyspnea on exertion associated with right chest wall pain that irradiated to ipsilateral shoulder. On examination, the patient presented with abdominal distension and a painful palpable mass in the right hypochondrium. Laboratory studies indicated leukocytosis and neutrophilia, while the liver tests were normal. Multiphase contrast-enhanced computed tomography scan showed a liver with an occupying hypodense mass in the right lobule and heterogeneous reinforcement in the periphery, a central necrotic component and satellite lesions in the 6th and 8th segments. Importantly, a 39 mm left auricular multi-lobulated mass associated with a lytic lesion was detected in the fourth right costal arch, both of which suggested secondary deposits (Fig. 1).
The patient was hospitalized for extensive work-up and initiated with intravenous antibiotics. Evaluation of intracardiac lesion included a transthoracic echocardiogram, which demonstrated a multinodular image attached to left auricle causing intermittent obstruction with a gradient of 32 mm/Hg. The right ventricle was dilated (43 mm) with tricuspid annular plane systolic excursion (TAPSE) of 18 mm suggestive of right heart failure and pulmonary hypertension confirmed by a pulmonary artery systolic pressure (PASP) of 87 mmHg. Biopsy of the hepatic lesion revealed no complications and revealed undifferentiated embryonal sarcoma. Throughout the follow-up, the patient manifested inflammatory response syndrome characterized by fever, leukocytosis, and tachycardia. No infectious focus was identified and the patient's urine and blood culture tested negative. Due to the poor prognosis, palliative radiotherapy and escalated intravenous analgesia were initiated without clinical improvement. Finally, the patient presented refractory hypoxemia resulting in cardiac arrest and death.
A 41-year-old woman presented to the emergency room with 1 month-long right upper quadrant abdominal pain associated with chills, nausea and emesis. Physical examination showed tachycardia and hepatomegaly with tenderness on palpation, and the remainder of the examination was normal. Laboratory studies showed normal levels of complete blood count and liver function parameters. Alpha-fetoprotein was within normal levels. An abdominal computed tomography showed a 20×16 cm-sized cystic lesion in the right hepatic lobule, with multiple septae and hypervascularization. Doppler ultrasound confirmed the finding and showed compression of intrahepatic vascular structures including portal vein (Fig. 2).
The patient underwent extended right hepatectomy without complications. Histopathological study reported high-grade undifferentiated embryonal sarcoma with negative margins and without lymphovascular and perineural invasion, or gangliona metastasis. The patient was discharged without pain and remained stable for two years, when a cystic lesion on the surgical site was documented during the follow-up. The lesion was located close to one of the main branches of the portal vein, and contraindicated for surgical resection.
Due to disease recurrence, chemotherapy with doxorubicin and isophosphamide was scheduled; however, only three cycles were administered due to hematological toxicity manifesting as neutropenia and fever. The lesion remained stable until three years when an increase in size and appearance of new lesions suggesting disease progression were observed (Fig. 3). After five years from the initial diagnosis, the patient was scheduled to receive chemotherapy with gemcitabine and docetaxel.
Patients with UESL usually present with an abdominal mass and abdominal pain, associated with anorexia, lethargy, and weight loss. Other symptoms may include nausea, vomiting, constipation, and respiratory distress secondary to compression caused by the tumor.5 Spontaneous rupture of the neoplasm has also been documented.6 Liver test results and neoplastic markers are usually normal. In patients with associated hemorrhage and necrosis inside the tumor, fever and increased levels of systemic inflammatory markers including C reactive protein, erythrocyte sedimentation rate, and leukocytosis, may be present.7
Liver tumors originate in hepatocytes, bile duct epithelium, neuroendocrine cells, and/or mesenchymal cells, which include fibroblasts, endothelium, adipocytes, myoblasts and chondroblasts.8 Macroscopically, UESL are single and well-circumscribed lesions with both cystic and solid components of gray-white gelatinous appearance and a fibrous pseudocapsule formed by compressed liver parenchyma. Frequently, dark-brown areas of hemorrhage and yellow-softer areas of necrosis are found.69 Microscopically, UESL shows undifferentiated spindle cells with ill-defined borders, inconspicuous nucleoli, hyperchromatic nuclei, and eosinophilic cytoplasm surrounded by a myxoid matrix showing undifferentiated and highly proliferative phenotype. Multinucleated giant cells and focal periodic acid Schiff-positive cytoplasmic inclusions resistant to diastase digestion were observed. Several mitotic figures are present as well.110 Immunohistochemically, UESL does not show a specific pattern. It may express histiocytic, muscle and epithelial markers, commonly vimentin and α1-antitrypsin, suggesting origin in primitive stem cells.1112 Ultrastructural studies under electron microscope of UESL revealed fibroblastic or fibrohistiocytic differentiation with rough endoplasmic reticulum cisternae and prominent electron-dense lysosomes and actin filaments in the cytoplasm.13 Lauwers et al.14 demonstrated a common cytogenetic alteration on chromosome 19 in a UESL arising from a mesenchymal hamartomas (MH), suggesting a connection between the two entities similar to UESL representing a malignant progression from MH.
Radiographically, UESL presents as a large solitary tumor with cystic appearance due to its high-water content in the myxoid stroma that may lead to a mistaken diagnosis of benign cystic lesion. Computed tomography (CT) shows a well-defined, low intensity, heterogeneous, multilocular, cystic tumor with gross septa, characterized by an enhancing solid compartment and a non-enhancing cystic compartment.15 Tumors with a solid compartment are usually accompanied by hemorrhage and necrosis that is clearly evident on ultrasound, displaying a honeycomb appearance.16 The presence of discordant imaging findings of solid lesion on ultrasound and cystic lesion on CT are suggestive of UESL. Magnetic resonance imaging (MRI) reveals a well circumscribed hypodense lesion with multiple hyperdense septations and water attenuation. Solid lesions commonly show contrast enhancement although mild and heterogeneous peripheral enhancement secondary to central necrosis and hemorrhage may be observed. Cystic lesions show low signal intensity on T1-weighted MRI and high signal intensity on T2-weighted images suggesting water content. Furthermore, areas with high signal intensity on T1-weighted images and low signal intensity on T2-weighted images suggest hemorrhage.1718
Surgical resection and chemotherapy are the primary treatment modalities for UESL, and chemotherapy is based on a combination of vincristine, actinomycin, ifosfamide and doxorubicin.19 In the absence of complete tumor resection at the time of diagnosis, definitive surgery may be indicated after neoadjuvant chemotherapy. The current 1-year and 2-year survival rates in patients with UESL are 61% and 55%, respectively, with a median survival of 29 months. However, recurrence is a common scenario reported in 32% of the cases with complete resection and adjuvant chemotherapy after a median follow-up of 28 months.20
Figures and Tables
Fig. 1
Computed tomography with intravenous contrast showing a (A) right liver lobule with a 20 cm-sized hypodense lesion (arrow) with irregular borders, a few septa, and centripetal reinforcement without any intra or extrahepatic biliary tract dilation. (B) The heart was augmented in size and the left auricle showed a 39 mm-sized hypodense mass with multi-lobulated borders leading to filling defect (black arrow). In addition, a lytic lesion in the fourth right costal arch was documented (white arrow), both of which suggesting secondary deposits.

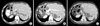
Fig. 2
Computed tomography with intravenous contrast that showed a (A) liver enlargement due to the presence of a 26 cm-sized cystic lesion with multiple thick septa (arrow). The lesion showed reinforcement with contrast material without evidence of solid tissue, in addition to compression and displacement of structures in the midline towards the left. Abdominal ultrasound (B) showed a previously described lesion with inner echoes and multiple septa, and compressed intrahepatic vascular structures including portal vein (arrow).
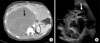
Fig. 3
Computed tomography with intravenous contrast (A) after extended right hepatectomy with post-surgical changes, the left lobule showed homogeneous density and regular form as well as an intrahepatic biliary tract with preserved caliber. Two years later (B) there was a 10 mm-sized hypodense heterogeneous lesion in the anterior portion of the left portal vein without occasional filling defects. The remainder of the liver parenchyma showed no changes. Five years after the initial diagnosis (C) the lesion documented previously showed an increase in size to 30 mm, suggesting disease progression.
References
1. Liver Cancer Study Group of Japan. Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann Surg. 1990; 211:277–287.
2. Stocker JT, Ishak KG. Undifferentiated (embryonal) sarcoma of the liver: report of 31 cases. Cancer. 1978; 42:336–348.
3. Jiménez Fuertes M, López Andújar R, de Juan Burgueño M, Moya Herráiz A, Sanjuán Rodríguez F, Montalvá Orón E, et al. Sarcoma indiferenciado (embrionario) de hígado del adulto: informe de un caso y revisión de la literatura médica. Gastroenterol Hepatol. 2008; 31:12–17.

4. Mori A, Fukase K, Masuda K, Sakata N, Mizuma M, Ohtsuka H, et al. A case of adult undifferentiated embryonal sarcoma of the liver successfully treated with right trisectionectomy: a case report. Surg Case Rep. 2017; 3:19.

5. Putra J, Ornvold K. Undifferentiated embryonal sarcoma of the liver: a concise review. Arch Pathol Lab Med. 2015; 139:269–273.

6. Sakellaridis T, Panagiotou I, Georgantas T, Micros G, Rontogianni D, Antiochos C. Undifferentiated embryonal sarcoma of the liver mimicking acute appendicitis. Case report and review of the literature. World J Surg Oncol. 2006; 4:9.
7. Wei ZG, Tang LF, Chen ZM, Tang HF, Li MJ. Childhood undifferentiated embryonal liver sarcoma: clinical features and immunohistochemistry analysis. J Pediatr Surg. 2008; 43:1912–1919.

8. Harman M, Nart D, Acar T, Elmas N. Primary mesenchymal liver tumors: radiological spectrum, differential diagnosis, and pathologic correlation. Abdom Imaging. 2015; 40:1316–1330.

9. Pachera S, Nishio H, Takahashi Y, Yokoyama Y, Oda K, Ebata T, et al. Undifferentiated embryonal sarcoma of the liver: case report and literature survey. J Hepatobiliary Pancreat Surg. 2008; 15:536–544.

10. Hong WJ, Kang YN, Kang KJ. Undifferentiated embryonal sarcoma in adult liver. Korean J Pathol. 2014; 48:311–314.

11. Lee KH, Maratovich MN, Lee KB. Undifferentiated embryonal sarcoma of the liver in an adult patient. Clin Mol Hepatol. 2016; 22:292–295.

12. Kiani B, Ferrell LD, Qualman S, Frankel WL. Immunohistochemical analysis of embryonal sarcoma of the liver. Appl Immunohistochem Mol Morphol. 2006; 14:193–197.

13. Agaram NP, Baren A, Antonescu CR. Pediatric and adult hepatic embryonal sarcoma: a comparative ultrastructural study with morphologic correlations. Ultrastruct Pathol. 2006; 30:403–408.

14. Lauwers GY, Grant LD, Donnelly WH, Meloni AM, Foss RM, Sanberg AA, et al. Hepatic undifferentiated (embryonal) sarcoma arising in a mesenchymal hamartoma. Am J Surg Pathol. 1997; 21:1248–1254.

15. Yang L, Chen LB, Xiao J, Han P. Clinical features and spiral computed tomography analysis of undifferentiated embryonic liver sarcoma in adults. J Dig Dis. 2009; 10:305–309.

16. Xie ZY, Li LP, Wu WJ, Sun DY, Zhou MH, Zhao YG. Undifferentiated embryonal sarcoma of the liver mistaken for hepatic abscess in an adult. Oncol Lett. 2014; 8:1184–1186.

17. Buetow PC, Buck JL, Pantongrag-Brown L, Marshall WH, Ros PR, Levine MS, et al. Undifferentiated (embryonal) sarcoma of the liver: pathologic basis of imaging findings in 28 cases. Radiology. 1997; 203:779–783.

18. Kim KA, Kim KW, Park SH, Jang SJ, Park MS, Kim PN, et al. Unusual mesenchymal liver tumors in adults: radiologic-pathologic correlation. AJR Am J Roentgenol. 2006; 187:W481–W489.

19. Lenze F, Birkfellner T, Lenz P, Hussein K, Länger F, Kreipe H, et al. Undifferentiated embryonal sarcoma of the liver in adults. Cancer. 2008; 112:2274–2282.

20. Bisogno G, Pilz T, Perilongo G, Ferrari A, Harms D, Ninfo V, et al. Undifferentiated sarcoma of the liver in childhood: a curable disease. Cancer. 2002; 94:252–257.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download