Abstract
Background
The aim of this study was to investigate the factors associated with resolution of hypertension after adrenalectomy in patients with primary aldosteronism. A secondary aim was to describe our use of the contralateral ratio in adrenal venous sampling (AVS) in the setting of suboptimal successful cannulation rates.
Methods
A retrospective review of patients who underwent AVS followed by unilateral adrenalectomy for primary aldosteronism was performed.
Results
Complete resolution of hypertension and hypokalemia was seen in 17 of 40 patients (42.5%), while a clinical improvement in hypertension was seen in 38 of 40 (95%). Shorter duration of hypertension, mean aldosteronoma resolution score (ARS), and a high ARS of 3 to 5 were associated with resolution of hypertension after adrenalectomy (P=0.02, P=0.02, and P=0.004, respectively). Of the individual components of ARS, only a duration of hypertension of ≤6 years was associated with resolution of hypertension after adrenalectomy (P=0.03).
Primary aldosteronism is being increasingly diagnosed in hypertensive patients. This may be related to an increased awareness of the need for screening, especially in patients with concomitant hypokalemia, and because adrenal incidentalomas are more commonly identified as imaging becomes a more routine part of patient care. The prevalence of primary aldosteronism is estimated to be 5% to 11% among individuals with hypertension [12345]. In a prospective study of hypertensive patients in Singapore, the prevalence of primary aldosteronism was estimated to be 5%, and hypokalemia was present in 37.5% of those patients [56].
Primary aldosteronism is an important cause of secondary hypertension, as it has been associated with worse cardiovascular complications than essential hypertension [7]. The causes of primary aldosteronism include aldosterone-producing adenoma (APA) (30% to 50%) [18], bilateral adrenal hyperplasia (50% to 65%) [18], and rarely, glucocorticoid-suppressible hyperaldosteronism, adrenocortical carcinoma, and primary adrenal hyperplasia. The management of unilateral adrenal hypersecretion (APA or primary adrenal hyperplasia) and bilateral adrenal hyperplasia are different and thus, the distinction between them is crucial.
Adrenal venous sampling (AVS) has been advocated as the reference test for the lateralization of aldosterone hypersecretion in patients with primary aldosteronism [910]. Using computed tomography (CT) imaging alone for deciding upon adrenalectomy in cases of unilateral aldosterone hypersecretion would lead to inappropriate surgery in 20% to 50% of patients [8111213]. However, AVS remains a technically challenging and operatordependent procedure, mainly due to difficulties in cannulation of the right adrenal vein. This is reflected in the widely varying rates of successful bilateral adrenal vein cannulations, which have been reported to be 8% to 96% at various centers [811,14151617]. The Adrenal Vein Sampling International Study showed that the use of AVS, even at large centers, was not routine or standardized worldwide [18]. Even with successful bilateral adrenal vein cannulations, significant confusion exists due to controversies regarding the appropriate cutoffs for the selectivity index, lateralization ratio (LR), and contralateral ratio (CR), as well as the use of cosyntropin during AVS. These problems highlight the necessity to identify patients who will benefit most from adrenalectomy before subjecting them to AVS and surgery.
The main aim of this paper was to investigate the factors associated with resolution of hypertension after adrenalectomy, including the aldosteronoma resolution score (ARS). We also described our experiences in using the CR in the setting of suboptimal AVS results in patients who underwent surgery.
The records and results of all patients who underwent AVS at Singapore General Hospital from January 1, 2003 to December 31, 2013 were reviewed retrospectively. The study was approved by Singapore General Hospital's Institutional Review Board who waived the requirement for informed written consent for this study. All patients had primary aldosteronism that was identified at screening based on a plasma aldosterone concentration (PAC) to plasma renin activity (PRA) ratio ≥20 and an elevated PAC (≥416 pmol/L) [9]. Confirmatory tests were performed with intravenous or oral salt loading tests. A PAC ≥277 pmol/L after infusion of 2 L of 0.9% saline was confirmatory of primary aldosteronism. For the oral salt loading test, the diagnosis of primary aldosteronism was confirmed if 24-hour urinary aldosterone was ≥39 nmol/day in the presence of a urinary sodium excretion of >250 mmol/day.
Electronic and written medical records were retrospectively reviewed to obtain baseline characteristics, the indication for testing for primary aldosteronism, the number of antihypertensives used, and laboratory, radiological, and histology results. Factors associated with resolution of hypertension after adrenalectomy were investigated. These factors included the ARS score [19], which consists of the following: number of antihypertensive medications ≤2 (2 points), body mass index (BMI) ≤25 kg/m2 (1 point), ≤6 years of hypertension (1 point), and female sex (1 point).
The clinical outcome data collected after adrenalectomy included potassium levels, PAC, and the number of antihypertensive medications at 6-month to 1-year postoperative follow-up examinations. Clinical improvement was defined as resolution of hypertension or reduction in the use of antihypertensives.
All patients underwent CT imaging of the adrenals, but dedicated adrenal CT was only performed in 71 patients. The remaining cases were adrenal incidentalomas detected on abdominal CT or a CT urogram.
Regardless of CT findings, all patients with primary aldosteronism who were deemed surgical candidates for adrenalectomy, were willing to undergo surgery, and agreed to undergo AVS underwent the procedure by interventional radiologists. At our center, AVS procedures were performed by a group of experienced interventional radiologists. If present, hypokalemia was corrected before AVS to avoid blunting of aldosterone secretion. An adrenocorticotropic hormone (ACTH) infusion (50 µg/hr) was used in 79 of 81 patients (97.5%). In the earlier years (2003 to 2004), ACTH infusions were not uniformly used, but were subsequently implemented into our institute's AVS protocol for standardization purposes. These two patients were excluded from our analysis (Fig. 1). Paired blood samples were obtained from the bilateral adrenal veins and inferior vena cava for cortisol and aldosterone measurements during sequential catheterization. Adrenal vein cannulation was regarded as successful if the adrenal-peripheral cortisol ratio (selectivity index) was >5. Positive lateralization was defined as LR ≥4. A CR <1 was considered positive. Prior to blood sampling from the adrenal veins, catheter placement was checked via fluoroscopy.
At the first attempt of AVS, bilateral cannulation was successful in 44 of 79 patients (55.6%). The options offered after unsuccessful AVS at the first attempt were repeat AVS, medical treatment, or unilateral adrenalectomy. Among the 36 patients in whom AVS was unsuccessful, only two agreed to undergo repeat AVS. One of these procedures was successful, resulting in a total of 45 patients (57%) who had successful AVS. The cannulation success rates were 46 of 79 (58.2%) on the right, and 74 of 79 (93.7%) on the left.
The patients with bilateral successful cannulation were divided into groups with various combinations of LR and CR (Fig. 1): Group A: positive LR and positive CR (n=20); Group B: positive CR but negative LR (n=8); Group C: positive LR but negative CR (n=1); Group D: negative LR and positive CR (n=6); and Group E: invalid LR or CR because aldosterone and cortisol assays were not diluted to absolute levels (n=10).
There was only one patient who had a negative CR with a positive LR. This patient did not undergo adrenalectomy. There were no cases of mortality from AVS, although one of the patient who underwent a repeat AVS had adrenal hemorrhage secondary to the AVS procedure.
All patients who did not undergo surgery received medical treatment with spironolactone or eplerenone.
Statistical analysis was performed using SPSS Statistics version 20.0 (IBM Corp., Armonk, NY, USA). In the univariate analysis, the chi-square test was used for categorical variables and the Student t test for continuous variables. P values <0.05 were considered to indicate statistical significance. All results are expressed as mean±standard error of the mean.
The baseline characteristics of the 40 patients who underwent surgery are summarized in Table 1. Their mean age was 52.8 years old, and 55% were male. The majority were of Chinese ethnicity (90%), while the rest were Indian and Malay (5% each).
The most common indication for screening for primary aldosteronism was hypertension with hypokalemia (80%). The other indications were adrenal incidentaloma (15%) and hypertension at a young age (5%). The mean LR was 14.8 (n=18), while the mean CR was 0.35 (n=27).
Thirty-seven patients (92.5%) had unilateral adrenal masses, with 60% found on the left and 32.5% on the right. Three patients had bilateral adrenal masses observed on CT. None of the patients who underwent surgery had a normal CT scan of the adrenals. The site of the adrenal adenoma on the CT scan was correctly correlated with the histology result from surgery in 36 of the 40 patients (90%). There were six incorrect correlations of the CT scan and AVS results; in these cases, AVS confirmed bilateral adrenal hyperplasia.
The histology results of all patients who underwent adrenalectomy was adenoma, except for two cases of cortical hyperplasia and one cavernous hemangioma. The patients who had cortical hyperplasia and cavernous hemangioma did show clinical improvements after adrenalectomy, but not resolution of hypertension.
Among all the patients that underwent adrenalectomy, there was a reduction of antihypertensives postoperatively. The mean number of preoperative and postoperative antihypertensives in this group was 2±0.1 and 0.8±0.1, respectively. The number of patients requiring three and four antihypertensives as reduced from eight of 40 (20%) and two of 40 (5%), respectively, to none. The number of patients requiring two antihypertensives was 17 of 40 (42.5%) preoperatively, and this was reduced to 10 of 40 (25%) postoperatively. Thirteen of the 40 patients (32.5%) required a single antihypertensive both preoperatively and postoperatively. The mean potassium level of the patients who underwent adrenalectomy also improved from 2.9±0.1 to 4.5±0.1 mmol/L.
Resolution of hypertension was seen in 17 of the 40 patients (42.5%), and a clinical improvement in hypertension was seen in 38 of the 40 patients (95%). Resolution of hypokalemia occurred in all patients except one who did not achieve resolution of hypertension after adrenalectomy. After adrenalectomy, renin-aldosterone biochemistry normalized in 23 of the 25 patients (92%) whose results were available.
A shorter duration of hypertension was significantly associated with resolution of hypertension after adrenalectomy (mean±SEM: 3.8±0.8 years vs. 8.5±1.5 years, P=0.02) (Table 2). A higher ARS of 3 to 5 was associated with a higher hypertension resolution rate (16 of 28; 57.1%) as compared to a lower ARS (0 to 2) (1 of 12; 8.3%; P=0.004). However, no association with resolution of hypertension was found when comparing an ARS of 4 to 5 to an ARS 0 to 3 or when comparing an ARS of 5 to an ARS of 0 to 4 (results not shown). Although a high ARS of 3 to 5 and a higher mean ARS were associated with resolution of hypertension after adrenalectomy (P=0.004 and P=0.02, respectively), the individual components of ARS were not statistically significantly associated with resolution of hypertension after adrenalectomy, except for a duration of hypertension ≤6 years (P=0.03). Neither the mean CR nor a CR ≤0.5 was associated with resolution of hypertension after adrenalectomy.
There were no significant differences in age, gender, race, BMI, baseline potassium levels, preoperative PRA, or preoperative PAC between patients who had resolution of hypertension after adrenalectomy versus those who did not.
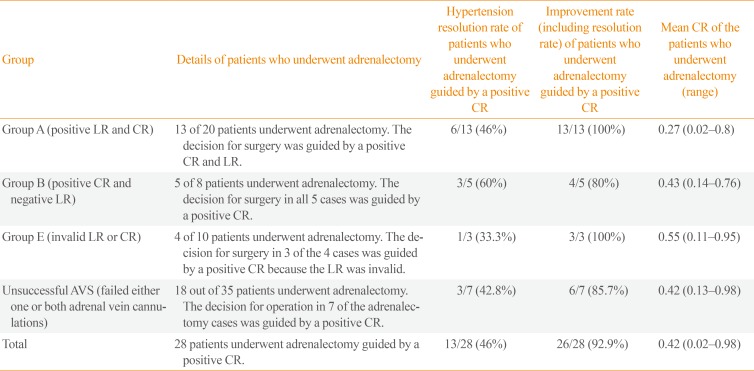
Of the 40 patients who underwent adrenalectomy for primary aldosteronism, the decision for surgery was guided by a positive CR in 28 (Table 3). Details of 81 patients who underwent AVS followed by surgery are described in Fig. 1.
In Group A (positive LR and positive CR), 100% showed a clinical improvement in blood pressure after adrenalectomy, of whom six of 13 (46%) had resolution of hypertension (Fig. 1).
In Group B (positive CR and negative LR), five of eight patients underwent surgery, and clinical improvement was observed in four of those five patients (80%). In this group, a positive CR (CR <1) was used to aid the decision for surgery. The LR was between 2 and 4. The patient without clinical improvement in hypertension after surgery had an LR of 2.83 with a CR of 0.69 and had hypertension for 20 years. Surgical resection of a 1.3-cm right adrenal nodule confirmed it to be adrenal adenoma.
In Group E (invalid LR or CR), among the four patients who underwent surgery, the decision for surgery in three patients was guided by a positive CR (range, 0.11 to 0.95) as the LR was invalid. Histology confirmed adrenal adenoma in all patients.
Of the 35 patients in whom AVS was unsuccessful, 18 (51.4%) underwent surgery. Among these 18 patients, seven in whom right-sided cannulation failed underwent right adrenalectomy, guided by a positive CR (0.13, 0.22, 0.22, 0.26, 0.46, 0.67, and 0.98) and right adrenal nodules on CT imaging. Clinical improvement after surgery was seen in six of seven patients (85.7%), three of whom had resolution of hypertension. Seventeen of the 18 patients who underwent adrenalectomy had clinical improvements in hypertension. The only patient who did not have clinical improvement was a 40-year-old ethnically Chinese man with young-onset hypertension and hypokalemia for 7 years. The adrenal CT scan showed a small right adrenal nodule, but with bilateral nodular and bulky adrenals. The CR was 0.98. The histology results showed two small right adrenocortical adenomas. The aldosterone-to-renin ratio was persistently high postoperatively and he was treated with eplerenone.
Zarnegar et al. [19] developed the ARS model, which has also been shown to have high predictive accuracy for postoperative cure in Japanese patients [20]. Many studies have found different factors to be associated with cure after adrenalectomy in their patient groups, including duration of hypertension, number of blood pressure medications, age, female sex, weight, size of the tumor, preoperative mineralocorticoid antagonist use, creatinine levels, and plasma aldosterone levels [212223242526272829]. However, not all components of the ARS model, apart from duration of hypertension, have been consistently found to be predictors of cure. In our study group, we found that a shorter duration of hypertension and a high ARS of 3 to 5 were associated with resolution of hypertension after adrenalectomy. Although a higher mean ARS and an ARS score of 3 to 5 were statistically significantly associated with hypertension resolution after adrenalectomy, of the four components of the ARS model, only duration of hypertension ≤6 years was associated with hypertension resolution. Using ≤2 antihypertensive medications, a BMI ≤25 kg/m2, and female sex each showed a positive association with hypertension resolution, although these associations were not statistically significant. This is very likely explained by the small numbers of patients in this study causing power limitations for the detection of statistical significance. Further studies in our local population are required to validate the use of ARS as a predictive tool. Apart from our study, a shorter duration of hypertension has been consistently found in many studies to be an important factor associated with hypertension resolution after adrenalectomy [19272829]. This is unsurprising, as chronic hyperaldosteronism causes irreversible cardiovascular and renal damage, leading to permanent hypertension [6].
The role of predictors of good after adrenalectomy outcomes is particularly important in the setting of suboptimal successful bilateral cannulation rates. In our large tertiary center, the rate of successful bilateral cannulation was lower, at 55.6%, than the success rates >90% that have been reported at some experienced tertiary centers. However, the German Conn's Registry illustrated a large divergence in success rates (with a mean rate of 30.5%), with centers doing fewer procedures having lower rates of success [17]. Cannulation of the right adrenal vein is technically more difficult due to its anatomical position. The small right adrenal vein drains directly into the inferior vena cava at different angles or directly into the small accessory hepatic vein [1030]. In contrast, the left adrenal vein, which drains into the left renal vein, is usually cannulated easily.
A significant difficulty arises when only one adrenal vein is successfully cannulated during AVS. Espiner et al. [31] suggested that assessment of the CR may be useful in such cases. The reasoning behind this is that if there is significant contralateral suppression of aldosterone secretion, the majority of aldosterone excess would presumably arise from the opposite adrenal gland. In cases where access was to a single adrenal vein contralateral to the site of unilateral hyperaldosteronism, the CR ranged from 0.07 to 0.4 [31]. In those with a unilateral lesion and bilateral successful cannulation, the mean CR was 0.54±0.16 (range, 0.1 to 1.4) [31]. A low CR has also been associated with primary aldosteronism cure in patients with an LR <4 on AVS who underwent unilateral adrenalectomy [32]. Strajina et al. [33] also showed that a CR ≤0.5 in patients with failed cannulation of one of the adrenal veins in AVS was predictive of unilateral primary aldosteronism. In our study, a CR ≤0.5 was not associated with hypertension resolution after surgery; however, our study is likely to be underpowered to detect any significance due to the lack of patients who underwent surgery with a CR >1 and the small sample size. There were 28 patients who had a positive CR, of whom 15 underwent surgery based on these CR results (CR <1): five cases with a negative LR, three cases with an invalid LR, and seven cases when cannulation of only one adrenal vein was successful. Of note, after adrenalectomy, 87% (13 of 15) of these patients had clinical improvement in hypertension.
The appropriate cut-off for CR still requires further study. In a study (without cosyntropin stimulation) of 93 patients in the UK, a CR <1.4 was found to be associated with unilateral aldosterone excess, which suggests that not all patients with APA have complete contralateral suppression [15]. Another published study of 99 patients also found that a CR <1.4 (with cosyntropin stimulation) was 94% specific and 90% sensitive for predicting lateralization [34]. Young et al. [8] also reported that 32% of bilateral adrenal hyperplasia cases had a CR <1, highlighting yet another limitation of the CR.
The use of the CR, however, has not been universally adopted. An expert consensus panel suggested that in general, AVS studies that are not bilaterally successful should not be used to establish lateralization. However, they acknowledged that in some instances, CR can be useful to guide clinical decisions when there are incomplete data for lateralization due to unsuccessful sampling. They cautioned, however, that adrenalectomy should only be done in cases in which the clinical likelihood of APA is high and that of persistent hypertension is low, as suggested by young age, a short duration of hypertension, a markedly high PAC and aldosterone-to-renin ratio, and normal renal function [10]. In our study, among five patients who underwent surgery based on AVS results of a positive CR and negative LR, there was one patient who did not achieve clinical improvement in hypertension. Although histology confirmed an adrenal adenoma and his hypokalemia resolved, he continued to require two antihypertensive medications, likely because of his long duration of hypertension (20 years) and co-existing essential hypertension. In cases such as this, evaluating predictors of cure after adrenalectomy would be very helpful to aid clinicians in management decisions, especially in weighing the risk versus benefit of surgery versus medical management.
Our study has several limitations. These include the retrospective study design and the small sample size. The successful AVS cannulation rate at our center was modest, but this is reflective of the real-world difficulties of AVS. Although our center uses a CR <1 as a guide to determine suitability for surgery in the setting of unilateral cannulation of the adrenal vein, the descriptive nature of our results do not allow a conclusion to be made regarding the exact role or cut-offs of CR. Our center did not have patients who underwent adrenalectomy who had a negative CR, and hence there is potential selection bias. Another limitation is that 37.5% of patients did not have their PAC and PRA levels checked after the operation to confirm the resolution of primary aldosteronism.
In conclusion, a shorter duration of hypertension and ARS of 3 to 5 were significantly associated with resolution of hypertension after adrenalectomy in patients with primary aldosteronism. Further studies are required to evaluate the role of CR in guiding the decision to perform adrenalectomy for primary aldosteronism in our local population.
References
1. Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006; 48:2293–2300. PMID: 17161262.

2. Lim PO, Rodgers P, Cardale K, Watson AD, MacDonald TM. Potentially high prevalence of primary aldosteronism in a primary-care population. Lancet. 1999; 353:40.

3. Kumar A, Lall SB, Ammini A, Peshin SS, Karmarkar MG, Talwar KK, et al. Screening of a population of young hypertensives for primary hyperaldosteronism. J Hum Hypertens. 1994; 8:731–732. PMID: 7807506.
4. Gordon RD, Stowasser M, Tunny TJ, Klemm SA, Rutherford JC. High incidence of primary aldosteronism in 199 patients referred with hypertension. Clin Exp Pharmacol Physiol. 1994; 21:315–318. PMID: 7923898.

5. Loh KC, Koay ES, Khaw MC, Emmanuel SC, Young WF Jr. Prevalence of primary aldosteronism among Asian hypertensive patients in Singapore. J Clin Endocrinol Metab. 2000; 85:2854–2859. PMID: 10946893.

6. Rossi GP, Sechi LA, Giacchetti G, Ronconi V, Strazzullo P, Funder JW. Primary aldosteronism: cardiovascular, renal and metabolic implications. Trends Endocrinol Metab. 2008; 19:88–90. PMID: 18314347.

7. Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005; 45:1243–1248. PMID: 15837256.

8. Young WF, Stanson AW, Thompson GB, Grant CS, Farley DR, van Heerden JA. Role for adrenal venous sampling in primary aldosteronism. Surgery. 2004; 136:1227–1235. PMID: 15657580.

9. Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008; 93:3266–3281. PMID: 18552288.

10. Rossi GP, Auchus RJ, Brown M, Lenders JW, Naruse M, Plouin PF, et al. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. 2014; 63:151–160. PMID: 24218436.

11. Oh EM, Lee KE, Yoon K, Kim SY, Kim HC, Youn YK. Value of adrenal venous sampling for lesion localization in primary aldosteronism. World J Surg. 2012; 36:2522–2527. PMID: 22736344.

12. Mathur A, Kemp CD, Dutta U, Baid S, Ayala A, Chang RE, et al. Consequences of adrenal venous sampling in primary hyperaldosteronism and predictors of unilateral adrenal disease. J Am Coll Surg. 2010; 211:384–390. PMID: 20800196.

13. Kempers MJ, Lenders JW, van Outheusden L, van der Wilt GJ, Schultze Kool LJ, Hermus AR, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med. 2009; 151:329–337. PMID: 19721021.

14. Minami I, Yoshimoto T, Hirono Y, Izumiyama H, Doi M, Hirata Y. Diagnostic accuracy of adrenal venous sampling in comparison with other parameters in primary aldosteronism. Endocr J. 2008; 55:839–846. PMID: 18497447.

15. Graham UM, Ellis PK, Hunter SJ, Leslie H, Mullan KR, Atkinson AB. 100 Cases of primary aldosteronism: careful choice of patients for surgery using adrenal venous sampling and CT imaging results in excellent blood pressure and potassium outcomes. Clin Endocrinol (Oxf). 2012; 76:26–32. PMID: 21767289.

16. Murashima M, Trerotola SO, Fraker DL, Han D, Townsend RR, Cohen DL. Adrenal venous sampling for primary aldosteronism and clinical outcomes after unilateral adrenalectomy: a single-center experience. J Clin Hypertens (Greenwich). 2009; 11:316–323. PMID: 19527322.

17. Vonend O, Ockenfels N, Gao X, Allolio B, Lang K, Mai K, et al. Adrenal venous sampling: evaluation of the German Conn's registry. Hypertension. 2011; 57:990–995. PMID: 21383311.
18. Rossi GP, Barisa M, Allolio B, Auchus RJ, Amar L, Cohen D, et al. The Adrenal Vein Sampling International Study (AVIS) for identifying the major subtypes of primary aldosteronism. J Clin Endocrinol Metab. 2012; 97:1606–1614. PMID: 22399502.

19. Zarnegar R, Young WF Jr, Lee J, Sweet MP, Kebebew E, Farley DR, et al. The aldosteronoma resolution score: predicting complete resolution of hypertension after adrenalectomy for aldosteronoma. Ann Surg. 2008; 247:511–518. PMID: 18376197.
20. Utsumi T, Kawamura K, Imamoto T, Kamiya N, Komiya A, Suzuki S, et al. High predictive accuracy of aldosteronoma resolution score in Japanese patients with aldosterone-producing adenoma. Surgery. 2012; 151:437–443. PMID: 22000827.

21. Carter Y, Roy M, Sippel RS, Chen H. Persistent hypertension after adrenalectomy for an aldosterone-producing adenoma: weight as a critical prognostic factor for aldosterone's lasting effect on the cardiac and vascular systems. J Surg Res. 2012; 177:241–247. PMID: 22921664.

22. Sawka AM, Young WF, Thompson GB, Grant CS, Farley DR, Leibson C, et al. Primary aldosteronism: factors associated with normalization of blood pressure after surgery. Ann Intern Med. 2001; 135:258–261. PMID: 11511140.

23. Steichen O, Zinzindohoue F, Plouin PF, Amar L. Outcomes of adrenalectomy in patients with unilateral primary aldosteronism: a review. Horm Metab Res. 2012; 44:221–227. PMID: 22395801.

24. Obara T, Ito Y, Okamoto T, Kanaji Y, Yamashita T, Aiba M, et al. Risk factors associated with postoperative persistent hypertension in patients with primary aldosteronism. Surgery. 1992; 112:987–993. PMID: 1455323.
25. Pang TC, Bambach C, Monaghan JC, Sidhu SB, Bune A, Delbridge LW, et al. Outcomes of laparoscopic adrenalectomy for hyperaldosteronism. ANZ J Surg. 2007; 77:768–773. PMID: 17685956.
26. Kupers EM, Amar L, Raynaud A, Plouin PF, Steichen O. A clinical prediction score to diagnose unilateral primary aldosteronism. J Clin Endocrinol Metab. 2012; 97:3530–3537. PMID: 22918872.
27. Zhang X, Zhu Z, Xu T, Shen Z. Factors affecting complete hypertension cure after adrenalectomy for aldosterone-producing adenoma: outcomes in a large series. Urol Int. 2013; 90:430–434. PMID: 23466491.

28. Giacchetti G, Ronconi V, Rilli S, Guerrieri M, Turchi F, Boscaro M. Small tumor size as favorable prognostic factor after adrenalectomy in Conn's adenoma. Eur J Endocrinol. 2009; 160:639–646. PMID: 19131503.

29. Tresallet C, Salepcioglu H, Godiris-Petit G, Hoang C, Girerd X, Menegaux F. Clinical outcome after laparoscopic adrenalectomy for primary hyperaldosteronism: the role of pathology. Surgery. 2010; 148:129–134. PMID: 20083287.

30. Davidson JK, Morley P, Hurley GD, Holford NG. Adrenal venography and ultrasound in the investigation of the adrenal gland: an analysis of 58 cases. Br J Radiol. 1975; 48:435–450. PMID: 1227698.

31. Espiner EA, Ross DG, Yandle TG, Richards AM, Hunt PJ. Predicting surgically remedial primary aldosteronism: role of adrenal scanning, posture testing, and adrenal vein sampling. J Clin Endocrinol Metab. 2003; 88:3637–3644. PMID: 12915648.

32. Umakoshi H, Tanase-Nakao K, Wada N, Ichijo T, Sone M, Inagaki N, et al. Importance of contralateral aldosterone suppression during adrenal vein sampling in the subtype evaluation of primary aldosteronism. Clin Endocrinol (Oxf). 2015; 83:462–467. PMID: 25727719.

33. Strajina V, Al-Hilli Z, Andrews JC, Bancos I, Thompson GB, Farley DR, et al. Primary aldosteronism: making sense of partial data sets from failed adrenal venous samplingsuppression of adrenal aldosterone production can be used in clinical decision making. Surgery. 2018; 163:801–806. PMID: 29174432.

34. Kline GA, Chin A, So B, Harvey A, Pasieka JL. Defining contralateral adrenal suppression in primary aldosteronism: implications for diagnosis and outcome. Clin Endocrinol (Oxf). 2015; 83:20–27. PMID: 25400021.

Fig. 1
Details of 81 patients who underwent adrenal venous sampling followed by surgery. ACTH, adrenocorticotropic hormone; AVS, adrenal venous sampling; LR, lateralization ratio; CR, contralateral ratio.
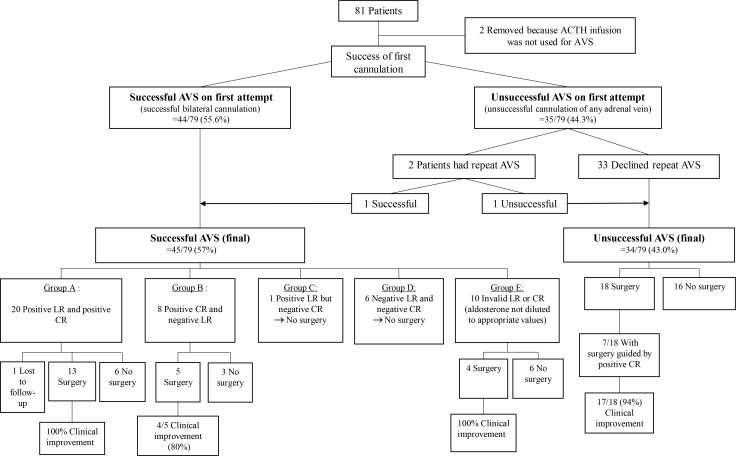
Table 1
Characteristics of the Patients Who Underwent Adrenalectomy for Primary Aldosteronism
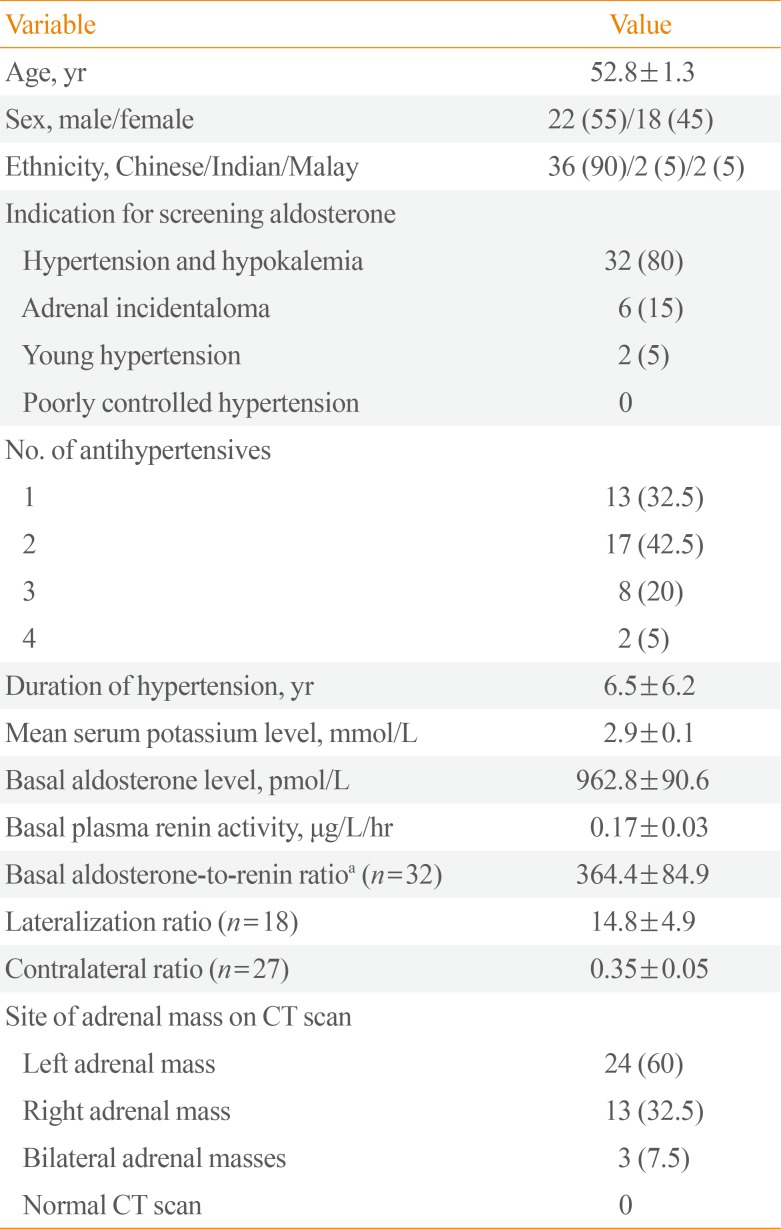
Table 2
Factors Associated with Resolution of Hypertension After Adrenalectomy in Patients with Primary Aldosteronism
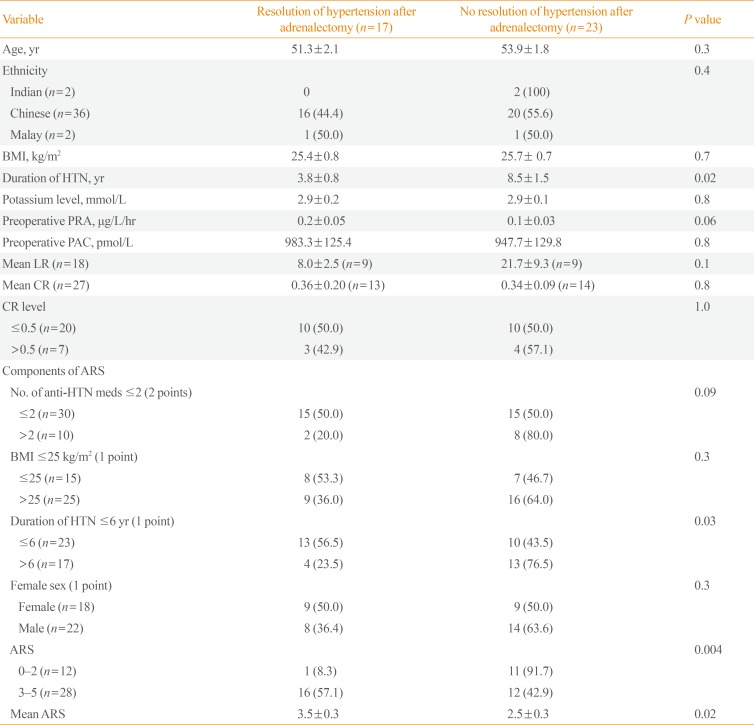
Table 3
Rates of Resolution and Improvement of Hypertension in Patients in Whom a Contralateral Ratio <1 Was Used to Assist in the Decision for Adrenalectomy




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download