Abstract
Type 2 diabetes (T2D) is associated with an increased risk of fracture, which has been reported in several epidemiological studies. However, bone mineral density in T2D is increased and underestimates the fracture risk. Common risk factors for fracture do not fully explain the increased fracture risk observed in patients with T2D. We propose that the pathogenesis of increased fracture risk in T2D is due to low bone turnover caused by osteocyte dysfunction resulting in bone microcracks and fractures. Increased levels of sclerostin may mediate the low bone turnover and may be a novel marker of increased fracture risk, although further research is needed. An impaired incretin response in T2D may also affect bone turnover. Accumulation of advanced glycosylation endproducts may also impair bone strength. Concerning antidiabetic medication, the glitazones are detrimental to bone health and associated with increased fracture risk, and the sulphonylureas may increase fracture risk by causing hypoglycemia. So far, the results on the effect of other antidiabetics are ambiguous. No specific guideline for the management of bone disease in T2D is available and current evidence on the effects of antiosteoporotic medication in T2D is sparse. The aim of this review is to collate current evidence of the pathogenesis, detection and treatment of diabetic bone disease.
Type 2 diabetes (T2D) is a prevalent disease currently affecting 420 million individuals worldwide with an expected increase to 629 million in the year 2045 [1]. Thus, prevention and intervention of complications is important to reduce morbidity and socioeconomic costs. A recently discovered complication of T2D is an increased risk of fractures, so-called diabetic bone disease. A number of previous meta-analyses have reported an increased risk of hip fractures in T2D of 1.4- to 1.7-fold [23]. More recent studies support this as both and Holm et al. [4] and Leslie et al. [5] report a 1.8 fold increase in hip fractures in T2D. However, not all studies report large increases in hip fracture risk. The study by Hothersall et al. [6] found merely a 1.05-fold increase in hip fracture risk in women with T2D compared to the general population and no increased hip fracture risk in men with T2D. A few additional studies reported no increased hip fracture risk in patients with T2D, but the number of patients with T2D are limited in these studies (ranging between 216 and 583) and may thus not have sufficient statistical power to detect hip fracture differences [789]. Besides hip fractures, the risk of low energy vertebral fracture is reported to be borderline significantly increased by 1.2-fold in a recent meta-analysis [10]. This finding is supported by the study by Napoli et al. [11] who reported a borderline significantly increased risk of vertebral fractures in patients with T2D after adjusting for bone mineral density (BMD). Furthermore, both fragility fractures and so-called low bone mass-related fractures are increased by 1.2-fold in patients with T2D [1012]. Patients with type 1 diabetes (T1D) may be included in some of the analyses if the diagnosis of T2D is based on information from registries. Thus, fracture risk may be overestimated and interpretation of the results should be conservative. However, limitations aside, T2D is associated with an increased risk of fractures—especially hip fractures. In a cross-sectional study, osteoporotic fractures impaired quality of life as much as other complications to diabetes [13]. Both hip fractures and vertebral fractures are related to increased mortality [1415]. Furthermore, the socioeconomic expenses related to fragility fractures in the European Union are major with an estimated expense of 37 billion euro in 2010 together with a significant loss of quality-adjusted life years [16]. As the population attributable risk of fractures will increase with the expected increasing prevalence of T2D [17], prevention of fractures is urgently needed in T2D. The aim of this review is to collate evidence of the pathogenesis, detection, and treatment of diabetic bone disease.
The increased fracture risk in patients with T2D may be due to a decreased bone quality. Bone quality is a compound entity which includes bone mass, bone turnover and bone material properties. However, in meta-analyses of BMD in patients with T2D compared to controls, BMD is increased and would thus mediate a relatively decreased fracture risk [318]. Hence, other mechanisms to the increased fracture risk in T2D apply than decreased BMD. As stated previously, bone material properties may be impaired. Few studies have investigated bone tissue biopsies from patients with T2D. Krakauer et al. [19] investigated five bone tissue biopsies and Manavalan et al. [20] four bone tissue biopsies from patients with T2D and both found evidence of low bone turnover in T2D. In an animal study, rats with T2D show evidence of decreased biomechanical efficiency and ductility of bone, which increase bone fragility at a given bone mass [21]. In another animal study, mice with T2D exhibited increased collagen maturity and bone mineral content compared to controls which is consistent with a reduced bone turnover and accumulation of old bone in patients with T2D [22]. It is suggested that advanced glycosylation endproducts (AGEs) produced in response to hyperglycemia are incorporated in the bone structure and reduce bone strength. Furthermore, AGEs may damage bone cells and alter bone turnover [23]. However, in a study assessing femoral neck biopsies from patients undergoing hip replacement, the AGE-content in neither bone nor serum was increased in patients with T2D compared with controls [24].
A meta-analysis from our group reports decreased bone turnover in patients with T2D based on bone turnover markers [25]. Levels of circulating bone resorption markers (tartrate resistant acid phosphatase and C-terminal cross-linked telopeptide of type-I collagen [CTX]) and bone formation markers (pro-collagen type I N-terminal propeptide [PINP] and osteocalcin) were lower in patients with T2D compared to controls, whereas circulating levels of the mineralization marker; bone specific alkaline phosphatase were similar to controls [25]. These findings suggest a relative hypermineralization in patients with T2D which could explain the higher BMD [26]. Low levels of bone turnover markers in T2D has also been reported in other meta-analysis [2728]. Bone turnover markers are lower in patients with T2D compared with patients with T1D [29]; thus, the low bone turnover-state may be specific to T2D. In our meta-analysis we also found increased levels of circulating sclerostin in patients with T2D compared with controls [25]. Circulating levels of sclerostin have previously been associated with low BMD and also correlated negatively with circulating levels of PINP in patients with T2D [30]. Sclerostin is produced by the osteocytes and regulate bone turnover by being an antagonist of the Wnt pathway [31]. The osteocytes respond to mechanical loading by reducing sclerostin production [32]. In T2D higher circulating levels of sclerostin were present in women with less than 2 hours walking activity daily compared to more walking activity [33]; thus, suggesting osteocytic dysfunction due to relative immobilization. Furthermore, when osteocyte-like cell lines are exposed to hyperglycemia, the sclerostin expression is increased [3435]. Taken together, osteocytic dysfunction may result in high levels of sclerostin and low bone turnover in patients with T2D. It has been suggested that the low bone turnover in T2D cause microcracks similar to what is observed in bisphosphonate therapy, thereby increasing fracture risk [36]. Bone material strength (BMS) index measured by micro-indentation is thought to reflect the resistance to microcrack generation [37]. In patients with T2D BMS is decreased compared to controls [3839]. The increased circulating sclerostin levels seem to be specific to T2D as it is not elevated in adult late autoimmune diabetes or in patients with T1D [4041].
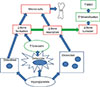
Hyperglycemia may directly influence osteoclasts and osteoblasts besides affecting the osteocytes. In vitro studies have shown that serum from patients with T2D inhibited the differentiation of mesenchymal stem cells to osteoblasts [42] and exposing human osteoblast-like cells to hyperglycemia for 7 and 14 days increased the matrix calcification with low quality mineral. However, the effect of hyperglycemia on osteoblasts in in vitro studies vary as osteocalcin production was decreased in some studies [43444546] and increased in other studies [4748]. In vitro studies of osteoclasts have demonstrated that hyperglycemia decreased osteoclast number, osteoclastogenesis, and osteoclast activity and decreased pit resorption has also been reported [495051]. The effects of hyperglycemia on osteoblasts and osteoclasts lead to hypermineralization and impaired resorption of mineralized matrix, which may contribute to the diabetic bone disease. Fig. 1 illustrates the hypothesis of ostecyte dysfunction and hypermineralization in T2D. Besides hyperglycemia and osteocytic dysfunction, insulin resistance has been correlated with low levels of bone turnover markers in patients with T2D [52] and the metabolic syndrome [53]. Furthermore, insulin resistance is correlated with decreased bone strength at the femoral neck measured by quantitative computed tomography (QCT) in healthy individuals in two studies [5455].
An impaired incretin response may also contribute to bone disease in diabetes. The incretins glucose-dependent insulinotropic peptide (GIP), glucagon-like peptide 1 (GLP-1), and GLP-2 are gastrointestinal hormones that are secreted postprandially with numerous beneficial effects on glucose metabolism. It is thought that incretins also mediate the reduction in bone turnover observed after feeding [56] as part of a gut-bone-axis. Receptors for GIP [57], GLP-1, and GLP-2 have been described in osteoblastic cell lines [58], GLP-1-receptors also in mature osteoblasts [59] and GIP-receptors in osteoclasts [60]. In patients with T2D, the effect of feeding on bone resorption is reduced compared with non-diabetes patients [61], possibly by an impaired GLP-1-response [62]. Further studies are warranted to investigate the role of incretins in bone turnover in T2D.
The changes in bone turnover may be reflected in the microarchitecture of the bone. However, a cross-sectional study with patients undergoing hip replacement surgery where samples were obtained from the femoral neck, no difference in cortical porosity or trabecular microstructure measured by microcomputed tomography was present compared to controls [24]. A limitation to the study was that only 20 patients with T2D where included. Non-invasive techniques may also be used to investigate the bone microarchitecture in T2D. Trabecular bone score (TBS) is a dual-energy X-ray absorptiometry (DXA)-derived variable which is thought to provide information about microarchitecture of the vertebrae and thereby improve fracture prediction in women with diabetes [63]. Women with T2D displayed low TBS compared to controls [6465] but in men, no difference in TBS was observed between T2D and controls [66]. TBS values have been negatively correlated to glycosylated hemoglobin A1c (HbA1c); thus, TBS may reflect the diabetic state [646667] but it remains to be shown if changes in HbA1c are reflected in changes in TBS. Furthermore, TBS was lower in patients with T2D and vertebral fracture compared to patients without fracture [68]. However, in the Fracture Risk Assessment Tool (FRAX) models adjusting by TBS does not fully capture the fracture risk in T2D [5]. Although TBS seems to have some value in detecting diabetic bone disease, current evidence does not support regular use of TBS and further research is needed to clarify whether TBS may add to current fracture prediction methods.
Measurement of cortical porosity by high resolution periphe-ral quantitative computed tomography (HRpQCT) has been proposed as a tool to identify patients with T2D at risk of fractures. However, cortical porosity has in different studies been reported to be either unchanged [38] or increased [697071] in patients with T2D compared to controls. In the mentioned studies the observed increased cortical porosity was possibly due to microvascular disease or previous fragility fractures. Furthermore, one study reports lower, higher, and unchanged cortical porosity in patients depending on the measured region [39]. A study using low-resolution computed tomography reports lower cortical porosity of the subtrochanteric femur in women with T2D compared to controls [72]. Besides cortical porosity HRpQCT evaluates trabecular and cortical structure and a study using HRpQCT reported an increased trabecular bone volume fraction in T2D [39] compared to controls, whereas other parameters were unchanged. Other studies report only cortical deficits [69] or unaltered microarchitecture in T2D compared with controls [38] or T1D [73], respectively. In another large study, HRpQCT revealed decreased cortical volumetric BMD at the tibia and increased cortical porosity at the radius in patients with T2D compared to controls [74]. The current evidence on cortical porosity and microstructure measured by HRpQCT in T2D is ambiguous and further research is needed to identify whether cortical porosity is a predictor of incident fractures.
QCT may discriminate vertebral fractures [75] and previous hip fractures [76] in non-diabetic individuals at a better rate than DXA and may thus be of use in patients with T2D. Heilmeier et al. [77] report that QCT may identify T2D patients with a previous fracture though the sample size in the study was limited (n=80). However, similar to issues with DXA the vertebral strength calculated from QCT did not correlate to prevalent vertebral fractures in patients with T2D [78]. Evidence on bone microarchitecture is limited in T2D and although several non-invasive assessments of bone microarchitecture have been performed in T2D with different techniques, the evidence does not currently support use of these assessments in detecting diabetic bone disease and further research is needed.
The fracture risk in patients with T2D is reported to be increased independent of age [679], whereas gender and body mass index influence fracture risk similarly to individuals without diabetes [79]. Obesity is related to T2D and has been associated with an increased fracture risk [80], but obesity was also associated with a decreased hip fracture risk in a meta-analysis of prospective studies [81]. Thus neither of these characteristics explain the fracture risk in patients with T2D. In patients with T2D, a weight loss of 20% or more leads to increased risk of fragility fractures compared to a weight loss of less than 10% [82]. Thus, changes in body composition may influence fracture risk and a rapid diet-induced weight loss is accompanied by increased circulating levels of sclerostin and CTX in patients with T2D [83]. Microangiopathy in diabetes is proposed to increase fracture risk by increasing cortical porosity [84] and diabetes complications may also influence fracture risk as peripheral neuropathy [8586], and retinopathy [987] is associated with an increased risk of fracture in T2D . However, in a registry based study, patients without diabetic complications also had an increased fracture risk and neither retinopathy nor neuropathy were associated with an increased risk of fractures [88]. Although diabetes complications as retinopathy and neuropathy may increase the risk of falls in T2D, it is uncertain how prominent the effect is on the increased risk of fracture in T2D [89]. Falls and osteoporotic fractures are associated in T2D [90]; however, fracture risk estimates that are adjusted by self-reported falls still demonstrate an increased risk of fractures in T2D [9192]. Also, hypoglycemia may cause falls, however fracture risk is still increased in T2D when adjusted for documented hypoglycemic episodes [93]. As described earlier, BMD does not fully explain the fracture risk in T2D, but Schwartz et al. [94] report that fracture risk is higher for a given T-score in T2D compared with controls. FRAX underestimates fracture risk in diabetes [95] and a correction factor by HbA1c has been proposed to enhance the prediction of fractures in patients with T2D [96]. Previous studies exploring the association between HbA1c and fracture risk have yielded conflicting results. High levels of HbA1c have been linked with an increased fracture risk in patients with diabetes [89798], but low levels of HbA1c below 7% have also been associated with an increased risk of hip fracture [99]. Some studies even reported that HbA1c is not associated with fracture risk [100]. In the randomized Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial no differences in falls or fracture were present between standard glycemic control (HbA1c, 7.5%) and intensive glycemic control (HbA1c, 6.4%) [101]. Overall, current fracture predictors underestimate fracture risk in patients with T2D; thus, there is a need for improved fracture predictors. In line with decreased bone turnover and increased sclerostin levels, sclerostin is being proposed as a fracture predictor in T2D and circulating levels of sclerostin have been associated with prevalent vertebral fractures [77102103]. However, it is uncertain whether sclerostin predicts incident fracture. Circulating levels of insulin like-growth factor-1 (IGF-1) have also been associated with an increased fracture risk [102104], both with prevalent vertebral fractures [102] and incident fractures [104]. Also, circulating levels of osteopontin and osteoglycin have been associated with prevalent vertebral fractures and may be novel fracture predictors in T2D [105106]. Future fracture predictors in T2D may be measurable in blood; however, further research is needed and until such fracture predictors are established, BMD continue to be the best fracture predictor available.
Multiple studies have investigated the effects of antidiabetic medication on fracture risk. Table 1 gives an overview of the effects of antidiabetic therapies on fracture risk. However, most studies are registry based and concerning many recently marketed drugs, the follow-up period may not be long enough yet to allow for the detection of fractures. Glitazones increase the risk of fractures as BMD is decreased by switching of osteoblastic recruitment to the adipocytic lineage [107108109]. This has been shown in both randomized controlled trials and observational studies [110111112]. Insulin use have shown both a neutral outcome on fracture risk [100113], increased fracture risk [114115116], and decreased fracture risk [93] in a number of different studies. Long-acting insulins may be less prone to cause hypoglycemia and have been associated with a decreased fracture risk compared to other insulins [117]. Animal studies have indicated a beneficial effect of sulphonylureas on bone by increasing bone formation, but so far no effect has been shown in humans [118]. Sulphonylureas are reported to have a neutral outcome on fracture risk [100113119], to increase fracture risk [120121122], and to decrease fracture risk [93]. Sulphonylureas increase the risk of hypoglycemia [123] and current use of sulphonylureas [110] and recent initiation [114] have been associated with an increased fracture risk in T2D, whereas ever use or cumulative dose have not been associated with increased fracture risk [110120]. Compared with metformin monotherapy insulin monotherapy and metformin and sulphonylurea in combination were associated with increased major osteoporotic fracture risk of 1.6- and 1.3-fold, respectively [114]. Metformin is first line therapy in the treatment of T2D. Cell and animal studies suggest metformin to be osteogenic by promoting Runx2 [124]; however, metformin is associated with lower circulating levels of PINP [125]. Metformin has generally been associated with a decreased or neutral effect on the risk of fractures in T2D [93100110112]. The severity of T2D determines whether second line therapy is added to the treatment and the effect of metformin found in observational studies may be due to confounding. More recent drugs as the dipeptidylpeptidase 4 (DPP-4) inhibitors, GLP-1 receptor agonists (GLP-1 RAs), and sodium-glucose co-transporter 2 (SGLT-2) inhibitors have also been investigated in terms of fracture risk. The DPP-4 inhibitors may have beneficial effects on fracture risk as DPP-4 inhibitor-use could potentiate a favorable effect of GLP-1 RA. Sitagliptin, a DPP-4 inhibitor, is in vitro shown to decrease osteoclastogenesis, whereas it is not known whether DPP-4 inhibitors affect osteoblasts [126]. A clinical study has shown that 1-year treatment with DPP-4 did not change postprandial levels of the bone resorption marker CTX nor calcium, phosphate, or serum alkaline phosphatase [127]. A number of retrospective studies have been conducted concerning DPP-4 inhibitors and fracture risk. One study showed that DPP-4 inhibitors were not associated with an increased fracture risk compared to sulphonylureas or insulin [113]. The DPP-4 inhibitors tended to reduce fracture risk compared to sulphonylureas with an hazard ratio of 0.8 (95% confidence interval, 0.51 to 1.24) and significantly reduced fracture risk compared to glitazones [113]. These effects may, however, represent harmful effects of sulphonylureas and glitazones. Ever use compared to no use of DPP-4 inhibitors have been associated with a decreased risk of fractures [128] and in a study with 5 years follow-up, use of DPP-4 inhibitors have been associated with a 14% decreased risk of fractures in patients with T2D compared to no use of DPP-4 inhibitors [129]. However in other cohort studies, DPP-4 inhibitors showed neutral effect with short treatment duration (less than an year) and longer treatment duration (4 years) [130131]. Concerning the different types of DPP-4 inhibitors, alogliptin was shown in a meta-analysis of randomized controlled trials to be associated with a lower risk of fracture compared to other DPP-4 inhibitors (saxagliptin and linagliptin) or placebo [132]. Although current evidence is ambiguous, studies with longer follow-up may reveal potential beneficial effects of DPP-4 inhibitors as most current studies are limited by a short treatment period. For GLP-1, as GLP-1 receptors are found on osteoblasts [58], GLP-1 is proposed to increase bone formation. In registry based studies and randomized controlled trials, GLP-1 RA were associated with neutral fracture risk. However, the GLP-1 RA treatment follow-up was too short to adequately evaluate fracture risk [133134135]. As stated previously, a large weight loss is associated with an increase in fracture risk [82]. GLP-1 RA may counteract this harmful effect of weight loss on fracture risk as GLP-1 RA-treatment has been shown to increase bone formation and reduce BMD-loss in obese non-diabetic women [136]. Further research and randomized controlled trials with a longer follow-up period is needed to determine the role of GLP-1 RA in fracture prevention in T2D. For the SGLT-2 inhibitors, concern has been raised as to whether the increased glucosuria may lead to loss of calcium and bone loss. Current evidence does not confirm the hypothesis of any harmful effects of SGLT-2 on fracture risk in randomized controlled trials [137138], although long-term effects are yet unknown. In conclusion, concerning the effect of antidiabetic therapies on diabetic bone disease caution should be applied to the use of glitazones in T2D in terms of fracture risk. Furthermore, sulphonylureas may through hypoglycemic events increase fracture risk which should be taken into account when selecting second-line antidiabetic drugs. Further research, investigating the effects of longer durations of treatment is needed on the more recently marketed antidiabetic drugs (DPP-4 inhibitors, GLP-1 RA, and SGLT-2 inhibitors). However, data from observational studies should be interpreted carefully as they are subject to residual confounding.
Few studies have investigated the effects of antiosteoporotic treatment in patients with T2D. A post hoc analysis of the Fracture Intervention Trial revealed increased BMD and decreased circulating levels of CTX and bone specific alkaline phosphatase in women with T2D randomized to antiresorptive treatment with the bisphosphonate alendronate [139]. Similar results are reported in a retrospective cohort [140]. A registry based study report similar effects of antiresorptive treatment in patients with T2D compared with controls [141]. Effects of antiresorptive treatment with denosumab on bone have not been investigated in patients with T2D. Studies on bone anabolic therapy with teriparatide report a reduction of fracture risk and increasing BMD in both patients with T2D and patients without diabetes [142]. A subgroup analysis of the recent VERtebral fracture treatment comparison in Osteoporotic women (VERO) trial comparing the effects of teriparatide with risedronate in women with severe osteoporosis demonstrated that the more pronounced anti-fracture efficacy of teriparatide was independent of prevalence of T2D [143].
Based on current evidence, antiosteoporotic therapy show similar effects in patients with and without T2D. However, concerning antiresorptive treatment, it can be speculated whether a further lowering of the already low bone turnover in T2D may be detrimental for bone quality and may actually increase fracture risk.
T2D is associated with an increased risk of fracture which may be due to detrimental effects of low bone turnover. Osteocytic dysfunction due to hyperglycemia, an impaired incretin response, factors related to hyperglycemia (e.g., insulin resistance) and low physical activity may all play a role in the development of diabetic bone disease. Furthermore, hyperglycemia may also cause hypermineralization relative to bone turnover and thus increase BMD and mask the actual fracture risk. Currently, BMD is the best predictor of fracture in T2D although flawed and biochemical markers such as sclerostin and IGF-1 should be evaluated further as potential markers for fracture risk in patients with T2D. In the clinical setting, awareness should be aimed at the avoiding the use of glitazones and specific antidiabetics that increase the risk of hypoglycemia (e.g., sulphonylureas) in order to reduce fractures in T2D. Currently, the knowledge of antiosteoporotic therapy in patients with T2D is sparse. More research on detection and treatment of diabetic bone disease and the associated increased risk of fractures is needed, but at present, current osteoporosis guidelines should be followed.
Figures and Tables
Fig. 1
The hypothesis of osteocyte dysfunction and hypermineralization. Hyperglycemia decreases bone resorption by inhibiting the osteoclast and decreases bone formation directly by inhibiting the osteoblast and indirectly by increasing sclerostin production by the osteocytes. The reduced bone turnover leads to microcracks and bone fractures. Furthermore, the hyperglycemia triggers hypermineralization in the bone causing high bone mineral density (BMD).
References
1. International Diabetes Federation. IDF diabetes atlas. 8th ed. Brussels: International Diabetes Federation;2017.
2. Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007; 166:495–505.

3. Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes: a meta-analysis. Osteoporos Int. 2007; 18:427–444.
4. Holm JP, Jensen T, Hyldstrup L, Jensen JB. Fracture risk in women with type II diabetes. Results from a historical cohort with fracture follow-up. Endocrine. 2018; 60:151–158.

5. Leslie WD, Johansson H, McCloskey EV, Harvey NC, Kanis JA, Hans D. Comparison of methods for improving fracture risk assessment in diabetes: the Manitoba BMD registry. J Bone Miner Res. 2018; 06. 28. [Epub]. DOI: 10.1002/jbmr.3538.

6. Hothersall EJ, Livingstone SJ, Looker HC, Ahmed SF, Cleland S, Leese GP, et al. Contemporary risk of hip fracture in type 1 and type 2 diabetes: a national registry study from Scotland. J Bone Miner Res. 2014; 29:1054–1060.

7. Dobnig H, Piswanger-Solkner JC, Roth M, Obermayer-Pietsch B, Tiran A, Strele A, et al. Type 2 diabetes mellitus in nursing home patients: effects on bone turnover, bone mass, and fracture risk. J Clin Endocrinol Metab. 2006; 91:3355–3363.

8. Oei L, Zillikens MC, Dehghan A, Buitendijk GH, Castano-Betancourt MC, Estrada K, et al. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: the Rotterdam Study. Diabetes Care. 2013; 36:1619–1628.

9. Ivers RQ, Cumming RG, Mitchell P, Peduto AJ. Blue Mountains Eye Study. Diabetes and risk of fracture: the blue mountains eye study. Diabetes Care. 2001; 24:1198–1203.

10. Jia P, Bao L, Chen H, Yuan J, Liu W, Feng F, et al. Risk of low-energy fracture in type 2 diabetes patients: a meta-analysis of observational studies. Osteoporos Int. 2017; 28:3113–3121.

11. Napoli N, Schwartz AV, Schafer AL, Vittinghoff E, Cawthon PM, Parimi N, et al. Vertebral fracture risk in diabetic elderly men: the MrOS study. J Bone Miner Res. 2018; 33:63–69.

12. Ni Y, Fan D. Diabetes mellitus is a risk factor for low bone mass-related fractures: a meta-analysis of cohort studies. Medicine (Baltimore). 2017; 96:e8811.
13. Voko Z, Gaspar K, Inotai A, Horvath C, Bors K, Speer G, et al. Osteoporotic fractures may impair life as much as the complications of diabetes. J Eval Clin Pract. 2017; 23:1375–1380.

14. Panula J, Pihlajamaki H, Mattila VM, Jaatinen P, Vahlberg T, Aarnio P, et al. Mortality and cause of death in hip fracture patients aged 65 or older: a population-based study. BMC Musculoskelet Disord. 2011; 12:105.

15. Hasserius R, Karlsson MK, Jonsson B, Redlund-Johnell I, Johnell O. Long-term morbidity and mortality after a clinically diagnosed vertebral fracture in the elderly: a 12- and 22-year follow-up of 257 patients. Calcif Tissue Int. 2005; 76:235–242.
16. Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark J, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. 2013; 8:136.

17. Starup-Linde J, Frost M, Vestergaard P, Abrahamsen B. Epidemiology of fractures in diabetes. Calcif Tissue Int. 2017; 100:109–121.

18. Ma L, Oei L, Jiang L, Estrada K, Chen H, Wang Z, et al. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol. 2012; 27:319–332.

19. Krakauer JC, McKenna MJ, Buderer NF, Rao DS, Whitehouse FW, Parfitt AM. Bone loss and bone turnover in diabetes. Diabetes. 1995; 44:775–782.

20. Manavalan JS, Cremers S, Dempster DW, Zhou H, Dworakowski E, Kode A, et al. Circulating osteogenic precursor cells in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012; 97:3240–3250.

21. Acevedo C, Sylvia M, Schaible E, Graham JL, Stanhope KL, Metz LN, et al. Contributions of material properties and structure to increased bone fragility for a given bone mass in the UCD-T2DM rat model of type 2 diabetes. J Bone Miner Res. 2018; 33:1066–1075.

22. Hunt HB, Pearl JC, Diaz DR, King KB, Donnelly E. Bone tissue collagen maturity and mineral content increase with sustained hyperglycemia in the KK-Ay murine model of type 2 diabetes. J Bone Miner Res. 2018; 33:921–929.

24. Karim L, Moulton J, Van Vliet M, Velie K, Robbins A, Malekipour F, et al. Bone microarchitecture, biomechanical properties, and advanced glycation end-products in the proximal femur of adults with type 2 diabetes. Bone. 2018; 114:32–39.

25. Hygum K, Starup-Linde J, Harslof T, Vestergaard P, Langdahl BL. Mechanisms in endocrinology: diabetes mellitus, a state of low bone turnover. A systematic review and meta-analysis. Eur J Endocrinol. 2017; 176:R137–R157.
26. Starup-Linde J, Vestergaard P. Biochemical bone turnover markers in diabetes mellitus: a systematic review. Bone. 2016; 82:69–78.
27. Purnamasari D, Puspitasari MD, Setiyohadi B, Nugroho P, Isbagio H. Low bone turnover in premenopausal women with type 2 diabetes mellitus as an early process of diabetes-associated bone alterations: a cross-sectional study. BMC Endocr Disord. 2017; 17:72.

28. Mitchell A, Fall T, Melhus H, Wolk A, Michaelsson K, Byberg L. Type 2 diabetes in relation to hip bone density, area, and bone turnover in Swedish men and women: a cross-sectional study. Calcif Tissue Int. 2018; 06. 26. [Epub]. DOI: 10.1007/s00223-018-0446-9.

29. Starup-Linde J, Lykkeboe S, Gregersen S, Hauge EM, Langdahl BL, Handberg A, et al. Differences in biochemical bone markers by diabetes type and the impact of glucose. Bone. 2016; 83:149–155.

30. Wang N, Xue P, Wu X, Ma J, Wang Y, Li Y. Role of sclerostin and dkk1 in bone remodeling in type 2 diabetic patients. Endocr Res. 2018; 43:29–38.

31. Manolagas SC, Almeida M. Gone with the Wnts: beta-catenin, T-cell factor, forkhead box O, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Mol Endocrinol. 2007; 21:2605–2614.
33. Raska I Jr, Raskova M, Zikan V, Skrha J. Prevalence and risk factors of osteoporosis in postmenopausal women with type 2 diabetes mellitus. Cent Eur J Public Health. 2017; 25:3–10.

34. Kang J, Boonanantanasarn K, Baek K, Woo KM, Ryoo HM, Baek JH, et al. Hyperglycemia increases the expression levels of sclerostin in a reactive oxygen species- and tumor necrosis factor-alpha-dependent manner. J Periodontal Implant Sci. 2015; 45:101–110.

35. Tanaka K, Yamaguchi T, Kanazawa I, Sugimoto T. Effects of high glucose and advanced glycation end products on the expressions of sclerostin and RANKL as well as apoptosis in osteocyte-like MLO-Y4-A2 cells. Biochem Biophys Res Commun. 2015; 461:193–199.

36. Farr JN, Khosla S. Determinants of bone strength and quality in diabetes mellitus in humans. Bone. 2016; 82:28–34.

38. Farr JN, Drake MT, Amin S, Melton LJ 3rd, McCready LK, Khosla S. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res. 2014; 29:787–795.

39. Nilsson AG, Sundh D, Johansson L, Nilsson M, Mellstrom D, Rudang R, et al. Type 2 diabetes mellitus is associated with better bone microarchitecture but lower bone material strength and poorer physical function in elderly women: a population-based study. J Bone Miner Res. 2017; 32:1062–1071.

40. Napoli N, Strollo R, Defeudis G, Leto G, Moretti C, Zampetti S, et al. Serum sclerostin and bone turnover in latent autoimmune diabetes in adults. J Clin Endocrinol Metab. 2018; 103:1921–1928.

41. Gennari L, Merlotti D, Valenti R, Ceccarelli E, Ruvio M, Pietrini MG, et al. Circulating sclerostin levels and bone turnover in type 1 and type 2 diabetes. J Clin Endocrinol Metab. 2012; 97:1737–1744.

42. Deng X, Xu M, Shen M, Cheng J. Effects of type 2 diabetic serum on proliferation and osteogenic differentiation of mesenchymal stem cells. J Diabetes Res. 2018; 2018:5765478.

43. Bartolome A, Lopez-Herradon A, Portal-Nunez S, Garcia-Aguilar A, Esbrit P, Benito M, et al. Autophagy impairment aggravates the inhibitory effects of high glucose on osteoblast viability and function. Biochem J. 2013; 455:329–337.
44. Botolin S, McCabe LR. Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J Cell Biochem. 2006; 99:411–424.

45. Wu YY, Yu T, Zhang XH, Liu YS, Li F, Wang YY, et al. 1,25(OH)2D3 inhibits the deleterious effects induced by high glucose on osteoblasts through undercarboxylated osteocalcin and insulin signaling. J Steroid Biochem Mol Biol. 2012; 132:112–119.

46. Zayzafoon M, Stell C, Irwin R, McCabe LR. Extracellular glucose influences osteoblast differentiation and c-Jun expression. J Cell Biochem. 2000; 79:301–310.

47. Liu Z, Jiang H, Dong K, Liu S, Zhou W, Zhang J, et al. Different concentrations of glucose regulate proliferation and osteogenic differentiation of osteoblasts via the PI3 kinase/Akt pathway. Implant Dent. 2015; 24:83–91.

48. Zhen D, Chen Y, Tang X. Metformin reverses the deleterious effects of high glucose on osteoblast function. J Diabetes Complications. 2010; 24:334–344.

49. Wittrant Y, Gorin Y, Woodruff K, Horn D, Abboud HE, Mohan S, et al. High d(+)glucose concentration inhibits RANKL-induced osteoclastogenesis. Bone. 2008; 42:1122–1130.

50. Xu J, Yue F, Wang J, Chen L, Qi W. High glucose inhibits receptor activator of nuclear factor-κB ligand-induced osteoclast differentiation via downregulation of v-ATPase V0 subunit d2 and dendritic cell specific transmembrane protein. Mol Med Rep. 2015; 11:865–870.
51. Xu F, Ye YP, Dong YH, Guo FJ, Chen AM, Huang SL. Inhibitory effects of high glucose/insulin environment on osteoclast formation and resorption in vitro. J Huazhong Univ Sci Technolog Med Sci. 2013; 33:244–249.

52. Tonks KT, White CP, Center JR, Samocha-Bonet D, Greenfield JR. Bone turnover is suppressed in insulin resistance, independent of adiposity. J Clin Endocrinol Metab. 2017; 102:1112–1121.

53. Laurent MR, Cook MJ, Gielen E, Ward KA, Antonio L, Adams JE, et al. Lower bone turnover and relative bone deficits in men with metabolic syndrome: a matter of insulin sensitivity? The European male ageing study. Osteoporos Int. 2016; 27:3227–3237.

54. Kalimeri M, Leek F, Wang NX, Koh HR, Roy NC, Cameron-Smith D, et al. Association of insulin resistance with bone strength and bone turnover in menopausal Chinese-Singaporean women without diabetes. Int J Environ Res Public Health. 2018; 15:E889.

55. Yang J, Hong N, Shim JS, Rhee Y, Kim HC. Association of insulin resistance with lower bone volume and strength index of the proximal femur in nondiabetic postmenopausal women. J Bone Metab. 2018; 25:123–132.

56. Clowes JA, Allen HC, Prentis DM, Eastell R, Blumsohn A. Octreotide abolishes the acute decrease in bone turnover in response to oral glucose. J Clin Endocrinol Metab. 2003; 88:4867–4873.

57. Bollag RJ, Zhong Q, Phillips P, Min L, Zhong L, Cameron R, et al. Osteoblast-derived cells express functional glucose-dependent insulinotropic peptide receptors. Endocrinology. 2000; 141:1228–1235.
58. Pacheco-Pantoja EL, Ranganath LR, Gallagher JA, Wilson PJ, Fraser WD. Receptors and effects of gut hormones in three osteoblastic cell lines. BMC Physiol. 2011; 11:12.

59. Nuche-Berenguer B, Portal-Nunez S, Moreno P, Gonzalez N, Acitores A, Lopez-Herradon A, et al. Presence of a functional receptor for GLP-1 in osteoblastic cells, independent of the cAMP-linked GLP-1 receptor. J Cell Physiol. 2010; 225:585–592.

60. Zhong Q, Itokawa T, Sridhar S, Ding KH, Xie D, Kang B, et al. Effects of glucose-dependent insulinotropic peptide on osteoclast function. Am J Physiol Endocrinol Metab. 2007; 292:E543–E548.

61. Chailurkit LO, Chanprasertyothin S, Rajatanavin R, Ongphiphadhanakul B. Reduced attenuation of bone resorption after oral glucose in type 2 diabetes. Clin Endocrinol (Oxf). 2008; 68:858–862.
62. Faerch K, Torekov SS, Vistisen D, Johansen NB, Witte DR, Jonsson A, et al. GLP-1 response to oral glucose is reduced in prediabetes, screen-detected type 2 diabetes, and obesity and influenced by sex: the ADDITION-PRO study. Diabetes. 2015; 64:2513–2525.

63. Leslie WD, Aubry-Rozier B, Lamy O, Hans D. Manitoba Bone Density Program. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013; 98:602–609.

64. Dhaliwal R, Cibula D, Ghosh C, Weinstock RS, Moses AM. Bone quality assessment in type 2 diabetes mellitus. Osteoporos Int. 2014; 25:1969–1973.

65. Rianon N, Ambrose CG, Buni M, Watt G, Reyes-Ortiz C, Lee M, et al. Trabecular bone score is a valuable addition to bone mineral density for bone quality assessment in older Mexican American women with type 2 diabetes. J Clin Densitom. 2018; 21:355–359.

66. Iki M, Fujita Y, Kouda K, Yura A, Tachiki T, Tamaki J, et al. Hyperglycemia is associated with increased bone mineral density and decreased trabecular bone score in elderly Japanese men: the Fujiwara-kyo osteoporosis risk in men (FORMEN) study. Bone. 2017; 105:18–25.

67. Kim JH, Choi HJ, Ku EJ, Kim KM, Kim SW, Cho NH, et al. Trabecular bone score as an indicator for skeletal deterioration in diabetes. J Clin Endocrinol Metab. 2015; 100:475–482.

68. Choi YJ, Ock SY, Jin Y, Lee JS, Kim SH, Chung Y. Urinary pentosidine levels negatively associates with trabecular bone scores in patients with type 2 diabetes mellitus. Osteoporos Int. 2018; 29:907–915.

69. Shanbhogue VV, Hansen S, Hermann P, Jorgensen N, Henriksen JE, Brixen K. Diabetic microvascular disease and bone structure in patients with type 2 diabetes mellitus. Diabetes. 2015; 64:Suppl 1. A174.
70. Patsch JM, Burghardt AJ, Yap SP, Baum T, Schwartz AV, Joseph GB, et al. Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J Bone Miner Res. 2013; 28:313–324.

71. Heilmeier U, Cheng K, Pasco C, Parrish R, Nirody J, Patsch JM, et al. Cortical bone laminar analysis reveals increased midcortical and periosteal porosity in type 2 diabetic postmenopausal women with history of fragility fractures compared to fracture-free diabetics. Osteoporos Int. 2016; 27:2791–2802.

72. Osima M, Kral R, Borgen TT, Hogestol IK, Joakimsen RM, Eriksen EF, et al. Women with type 2 diabetes mellitus have lower cortical porosity of the proximal femoral shaft using low-resolution CT than nondiabetic women, and increasing glucose is associated with reduced cortical porosity. Bone. 2017; 97:252–260.

73. Starup-Linde J, Lykkeboe S, Gregersen S, Hauge EM, Langdahl BL, Handberg A, et al. Bone structure and predictors of fracture in type 1 and type 2 diabetes. J Clin Endocrinol Metab. 2016; 101:928–936.

74. Samelson EJ, Demissie S, Cupples LA, Zhang X, Xu H, Liu CT, et al. Diabetes and deficits in cortical bone density, microarchitecture, and bone size: Framingham HR-pQCT study. J Bone Miner Res. 2018; 33:54–62.

75. Li N, Li XM, Xu L, Sun WJ, Cheng XG, Tian W. Comparison of QCT and DXA: osteoporosis detection rates in postmenopausal women. Int J Endocrinol. 2013; 2013:895474.

76. Yang L, Udall WJ, McCloskey EV, Eastell R. Distribution of bone density and cortical thickness in the proximal femur and their association with hip fracture in postmenopausal women: a quantitative computed tomography study. Osteoporos Int. 2014; 25:251–263.

77. Heilmeier U, Carpenter DR, Patsch JM, Harnish R, Joseph GB, Burghardt AJ, et al. Volumetric femoral BMD, bone geometry, and serum sclerostin levels differ between type 2 diabetic postmenopausal women with and without fragility fractures. Osteoporos Int. 2015; 26:1283–1293.

78. Kiyohara N, Yamamoto M, Sugimoto T. Discordance between prevalent vertebral fracture and vertebral strength estimated by the finite element method based on quantitative computed tomography in patients with type 2 diabetes mellitus. PLoS One. 2015; 10:e0144496.

79. Leslie WD, Morin SN, Lix LM, Majumdar SR. Does diabetes modify the effect of FRAX risk factors for predicting major osteoporotic and hip fracture? Osteoporos Int. 2014; 25:2817–2824.

80. Sogaard AJ, Holvik K, Omsland TK, Tell GS, Dahl C, Schei B, et al. Abdominal obesity increases the risk of hip fracture. A population-based study of 43,000 women and men aged 60-79 years followed for 8 years. Cohort of Norway. J Intern Med. 2015; 277:306–317.
81. Tang X, Liu G, Kang J, Hou Y, Jiang F, Yuan W, et al. Obesity and risk of hip fracture in adults: a meta-analysis of prospective cohort studies. PLoS One. 2013; 8:e55077.

82. Komorita Y, Iwase M, Fujii H, Ohkuma T, Ide H, Jodai-Kitamura T, et al. Impact of body weight loss from maximum weight on fragility bone fractures in Japanese patients with type 2 diabetes: the Fukuoka diabetes registry. Diabetes Care. 2018; 41:1061–1067.

83. Strollo R, Soare A, Manon Khazrai Y, Di Mauro A, Palermo A, Del Toro R, et al. Increased sclerostin and bone turnover after diet-induced weight loss in type 2 diabetes: a post hoc analysis of the MADIAB trial. Endocrine. 2017; 56:667–664.

84. Shanbhogue VV, Hansen S, Frost M, Brixen K, Hermann AP. Bone disease in diabetes: another manifestation of microvascular disease? Lancet Diabetes Endocrinol. 2017; 5:827–838.

85. Lee RH, Sloane R, Pieper C, Lyles KW, Adler RA, Van Houtven C, et al. Clinical fractures among older men with diabetes are mediated by diabetic complications. J Clin Endocrinol Metab. 2018; 103:281–287.

86. Kim JH, Jung MH, Lee JM, Son HS, Cha BY, Chang SA. Diabetic peripheral neuropathy is highly associated with nontraumatic fractures in Korean patients with type 2 diabetes mellitus. Clin Endocrinol (Oxf). 2012; 77:51–55.

87. Viegas M, Costa C, Lopes A, Griz L, Medeiro MA, Bandeira F. Prevalence of osteoporosis and vertebral fractures in postmenopausal women with type 2 diabetes mellitus and their relationship with duration of the disease and chronic complications. J Diabetes Complications. 2011; 25:216–221.
88. Vestergaard P, Rejnmark L, Mosekilde L. Diabetes and its complications and their relationship with risk of fractures in type 1 and 2 diabetes. Calcif Tissue Int. 2009; 84:45–55.

89. Timar B, Timar R, Gaita L, Oancea C, Levai C, Lungeanu D. The impact of diabetic neuropathy on balance and on the risk of falls in patients with type 2 diabetes mellitus: a cross-sectional study. PLoS One. 2016; 11:e0154654.

90. Yokomoto-Umakoshi M, Kanazawa I, Kondo S, Sugimoto T. Association between the risk of falls and osteoporotic fractures in patients with type 2 diabetes mellitus. Endocr J. 2017; 64:727–734.

91. Bonds DE, Larson JC, Schwartz AV, Strotmeyer ES, Robbins J, Rodriguez BL, et al. Risk of fracture in women with type 2 diabetes: the Women's Health Initiative Observational Study. J Clin Endocrinol Metab. 2006; 91:3404–3410.

92. Schwartz AV, Sellmeyer DE, Ensrud KE, Cauley JA, Tabor HK, Schreiner PJ, et al. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001; 86:32–38.

93. Vestergaard P, Rejnmark L, Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia. 2005; 48:1292–1299.

94. Schwartz AV, Vittinghoff E, Bauer DC, Hillier TA, Strotmeyer ES, Ensrud KE, et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA. 2011; 305:2184–2192.

95. Giangregorio LM, Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, et al. FRAX underestimates fracture risk in patients with diabetes. J Bone Miner Res. 2012; 27:301–308.

96. Valentini A, Cianfarani MA, De Meo L, Morabito P, Romanello D, Tarantino U, et al. FRAX tool in type 2 diabetic subjects: the use of HbA(1c) in estimating fracture risk. Acta Diabetol. 2018; 07. 06. [Epub]. DOI: 10.1007/s00592-018-1187-y.

97. Conway BN, Long DM, Figaro MK, May ME. Glycemic control and fracture risk in elderly patients with diabetes. Diabetes Res Clin Pract. 2016; 115:47–53.

98. Li CI, Liu CS, Lin WY, Meng NH, Chen CC, Yang SY, et al. Glycated hemoglobin level and risk of hip fracture in older people with type 2 diabetes: a competing risk analysis of Taiwan diabetes cohort study. J Bone Miner Res. 2015; 30:1338–1346.

99. Puar TH, Khoo JJ, Cho LW, Xu Y, Chen YT, Chuo AM, et al. Association between glycemic control and hip fracture. J Am Geriatr Soc. 2012; 60:1493–1497.

100. Starup-Linde J, Gregersen S, Vestergaard P. Associations with fracture in patients with diabetes: a nested case-control study. BMJ Open. 2016; 6:e009686.

101. Schwartz AV, Margolis KL, Sellmeyer DE, Vittinghoff E, Ambrosius WT, Bonds DE, et al. Intensive glycemic control is not associated with fractures or falls in the ACCORD randomized trial. Diabetes Care. 2012; 35:1525–1531.

102. Ardawi MS, Akhbar DH, Alshaikh A, Ahmed MM, Qari MH, Rouzi AA, et al. Increased serum sclerostin and decreased serum IGF-1 are associated with vertebral fractures among postmenopausal women with type-2 diabetes. Bone. 2013; 56:355–362.

103. Yamamoto M, Yamauchi M, Sugimoto T. Elevated sclerostin levels are associated with vertebral fractures in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013; 98:4030–4037.

104. Miyake H, Kanazawa I, Sugimoto T. Decreased serum insulin-like growth factor-I is a risk factor for non-vertebral fractures in diabetic postmenopausal women. Intern Med. 2017; 56:269–273.

105. Tanaka KI, Kanazawa I, Kaji H, Sugimoto T. Association of osteoglycin and FAM5C with bone turnover markers, bone mineral density, and vertebral fractures in postmenopausal women with type 2 diabetes mellitus. Bone. 2017; 95:5–10.

106. Filardi T, Carnevale V, Massoud R, Russo C, Nieddu L, Tavaglione F, et al. High serum osteopontin levels are associated with prevalent fractures and worse lipid profile in post-menopausal women with type 2 diabetes. J Endocrinol Invest. 2018; 06. 18. [Epub]. DOI: 10.1007/s40618-018-0914-0.

107. Meier C, Schwartz AV, Egger A, Lecka-Czernik B. Effects of diabetes drugs on the skeleton. Bone. 2016; 82:93–100.

108. Palermo A, D'Onofrio L, Eastell R, Schwartz AV, Pozzilli P, Napoli N. Oral anti-diabetic drugs and fracture risk, cut to the bone: safe or dangerous? A narrative review. Osteoporos Int. 2015; 26:2073–2089.

109. Harslof T, Wamberg L, Moller L, Stodkilde-Jorgensen H, Ringgaard S, Pedersen SB, et al. Rosiglitazone decreases bone mass and bone marrow fat. J Clin Endocrinol Metab. 2011; 96:1541–1548.
110. Starup-Linde J, Gregersen S, Frost M, Vestergaard P. Use of glucose-lowering drugs and risk of fracture in patients with type 2 diabetes. Bone. 2017; 95:136–142.

111. Bazelier MT, de Vries F, Vestergaard P, Herings RM, Gallagher AM, Leufkens HG, et al. Risk of fracture with thiazolidinediones: an individual patient data meta-analysis. Front Endocrinol (Lausanne). 2013; 4:11.

112. Kahn SE, Zinman B, Lachin JM, Haffner SM, Herman WH, Holman RR, et al. Rosiglitazone-associated fractures in type 2 diabetes: an Analysis from A Diabetes Outcome Progression Trial (ADOPT). Diabetes Care. 2008; 31:845–851.

113. Gamble JM, Donnan JR, Chibrikov E, Twells LK, Midodzi WK, Majumdar SR. The risk of fragility fractures in new users of dipeptidyl peptidase-4 inhibitors compared to sulfonylureas and other anti-diabetic drugs: a cohort study. Diabetes Res Clin Pract. 2018; 136:159–167.

114. Losada E, Soldevila B, Ali MS, Martinez-Laguna D, Nogues X, Puig-Domingo M, et al. Real-world antidiabetic drug use and fracture risk in 12,277 patients with type 2 diabetes mellitus: a nested case-control study. Osteoporos Int. 2018; 29:2079–2086.

115. Losada-Grande E, Hawley S, Soldevila B, Martinez-Laguna D, Nogues X, Diez-Perez A, et al. Insulin use and excess fracture risk in patients with type 2 diabetes: a propensity-matched cohort analysis. Sci Rep. 2017; 7:3781.

116. Wallander M, Axelsson KF, Nilsson AG, Lundh D, Lorentzon M. Type 2 diabetes and risk of hip fractures and non-skeletal fall injuries in the elderly: a study from the fractures and fall injuries in the elderly cohort (FRAILCO). J Bone Miner Res. 2017; 32:449–460.

117. Pscherer S, Kostev K, Dippel FW, Rathmann W. Fracture risk in patients with type 2 diabetes under different antidiabetic treatment regimens: a retrospective database analysis in primary care. Diabetes Metab Syndr Obes. 2016; 9:17–23.
118. Fronczek-Sokol J, Pytlik M. Effect of glimepiride on the skeletal system of ovariectomized and non-ovariectomized rats. Pharmacol Rep. 2014; 66:412–417.
119. Lapane KL, Yang S, Brown MJ, Jawahar R, Pagliasotti C, Rajpathak S. Sulfonylureas and risk of falls and fractures: a systematic review. Drugs Aging. 2013; 30:527–547.

120. Colhoun HM, Livingstone SJ, Looker HC, Morris AD, Wild SH, Lindsay RS, et al. Hospitalised hip fracture risk with rosiglitazone and pioglitazone use compared with other glucose-lowering drugs. Diabetologia. 2012; 55:2929–2937.

121. Napoli N, Strotmeyer ES, Ensrud KE, Sellmeyer DE, Bauer DC, Hoffman AR, et al. Fracture risk in diabetic elderly men: the MrOS study. Diabetologia. 2014; 57:2057–2065.

122. Rajpathak SN, Fu C, Brodovicz KG, Engel SS, Lapane K. Sulfonylurea use and risk of hip fractures among elderly men and women with type 2 diabetes. Drugs Aging. 2015; 32:321–327.

123. Schopman JE, Simon AC, Hoefnagel SJ, Hoekstra JB, Scholten RJ, Holleman F. The incidence of mild and severe hypoglycaemia in patients with type 2 diabetes mellitus treated with sulfonylureas: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2014; 30:11–22.

124. Molinuevo MS, Schurman L, McCarthy AD, Cortizo AM, Tolosa MJ, Gangoiti MV, et al. Effect of metformin on bone marrow progenitor cell differentiation: in vivo and in vitro studies. J Bone Miner Res. 2010; 25:211–221.

125. Stage TB, Christensen MH, Jorgensen NR, Beck-Nielsen H, Brosen K, Gram J, et al. Effects of metformin, rosiglitazone and insulin on bone metabolism in patients with type 2 diabetes. Bone. 2018; 112:35–41.

126. Wang C, Xiao F, Qu X, Zhai Z, Hu G, Chen X, et al. Sitagliptin, an anti-diabetic drug, suppresses estrogen deficiency-induced osteoporosisin vivo and inhibits RANKL-induced osteoclast formation and bone resorption in vitro. Front Pharmacol. 2017; 8:407.

127. Bunck MC, Poelma M, Eekhoff EM, Schweizer A, Heine RJ, Nijpels G, et al. Effects of vildagliptin on postprandial markers of bone resorption and calcium homeostasis in recently diagnosed, well-controlled type 2 diabetes patients. J Diabetes. 2012; 4:181–185.

128. Dombrowski S, Kostev K, Jacob L. Use of dipeptidyl peptidase-4 inhibitors and risk of bone fracture in patients with type 2 diabetes in Germany: a retrospective analysis of real-world data. Osteoporos Int. 2017; 28:2421–2428.
129. Hou WH, Chang KC, Li CY, Ou HT. Dipeptidyl peptidase-4 inhibitor use is associated with decreased risk of fracture in patients with type 2 diabetes: a population-based cohort study. Br J Clin Pharmacol. 2018; 84:2029–2039.

130. Driessen JH, van den Bergh JP, van Onzenoort HA, Henry RM, Leufkens HG, de Vries F. Long-term use of dipeptidyl peptidase-4 inhibitors and risk of fracture: a retrospective population-based cohort study. Diabetes Obes Metab. 2017; 19:421–428.

131. Driessen JH, van Onzenoort HA, Starup-Linde J, Henry R, Neef C, van den Bergh J, et al. Use of dipeptidyl peptidase 4 inhibitors and fracture risk compared to use of other anti-hyperglycemic drugs. Pharmacoepidemiol Drug Saf. 2015; 24:1017–1025.

132. Yang J, Huang C, Wu S, Xu Y, Cai T, Chai S, et al. The effects of dipeptidyl peptidase-4 inhibitors on bone fracture among patients with type 2 diabetes mellitus: a network meta-analysis of randomized controlled trials. PLoS One. 2017; 12:e0187537.

133. Driessen JH, van Onzenoort HA, Starup-Linde J, Henry R, Burden AM, Neef C, et al. Use of glucagon-like-peptide 1 receptor agonists and risk of fracture as compared to use of other anti-hyperglycemic drugs. Calcif Tissue Int. 2015; 97:506–515.

134. Driessen JH, de Vries F, van Onzenoort H, Harvey NC, Neef C, van den Bergh JP, et al. The use of incretins and fractures: a meta-analysis on population-based real life data. Br J Clin Pharmacol. 2017; 83:923–926.
135. Mabilleau G, Mieczkowska A, Chappard D. Use of glucagon-like peptide-1 receptor agonists and bone fractures: a meta-analysis of randomized clinical trials. J Diabetes. 2014; 6:260–266.
136. Iepsen EW, Lundgren JR, Hartmann B, Pedersen O, Hansen T, Jorgensen NR, et al. GLP-1 receptor agonist treatment increases bone formation and prevents bone loss in weight-reduced obese women. J Clin Endocrinol Metab. 2015; 100:2909–2917.

137. Tang HL, Li DD, Zhang JJ, Hsu YH, Wang TS, Zhai SD, et al. Lack of evidence for a harmful effect of sodium-glucose co-transporter 2 (SGLT2) inhibitors on fracture risk among type 2 diabetes patients: a network and cumulative meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2016; 18:1199–1206.

138. Kohler S, Kaspers S, Salsali A, Zeller C, Woerle HJ. Analysis of fractures in patients with type 2 diabetes treated with empagliflozin in pooled data from placebo-controlled trials and a head-to-head study versus glimepiride. Diabetes Care. 2018; 41:1809–1816.

139. Keegan TH, Schwartz AV, Bauer DC, Sellmeyer DE, Kelsey JL. fracture intervention trial. Effect of alendronate on bone mineral density and biochemical markers of bone turnover in type 2 diabetic women: the fracture intervention trial. Diabetes Care. 2004; 27:1547–1553.

140. Iwamoto J, Sato Y, Uzawa M, Takeda T, Matsumoto H. Three-year experience with alendronate treatment in postmenopausal osteoporotic Japanese women with or without type 2 diabetes. Diabetes Res Clin Pract. 2011; 93:166–173.
141. Vestergaard P, Rejnmark L, Mosekilde L. Are antiresorptive drugs effective against fractures in patients with diabetes? Calcif Tissue Int. 2011; 88:209–214.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download