1. Colović MB, Krstić DZ, Lazarević-Pašti TD, Bondžić AM, Vasić VM. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol. 2013; 11:315–335. DOI:
10.2174/1570159X11311030006. PMID:
24179466.
2. Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M. Alzheimer's disease: clinical trials and drug development. Lancet Neurol. 2010; 9:702–716. DOI:
10.1016/S1474-4422(10)70119-8. PMID:
20610346.

3. Shigeta M, Homma A. Donepezil for Alzheimer's disease: pharmacodynamic, pharmacokinetic, and clinical profiles. CNS Drug Rev. 2001; 7:353–368. PMID:
11830754.

4. Yasui-Furukori N, Furuya R, Takahata T, Tateishi T. Determination of donepezil, an acetylcholinesterase inhibitor, in human plasma by high-performance liquid chromatography with ultraviolet absorbance detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2002; 768:261–265. DOI:
10.1016/S1570-0232(01)00592-X.

5. Rogers SL, Friedhoff LT. Pharmacokinetic and pharmacodynamic profile of donepezil HCl following single oral doses. Br J Clin Pharmacol. 1998; 46(Suppl 1):1–6. DOI:
10.1046/j.1365-2125.1998.0460s1001.x.

6. Nakashima K, Itoh K, Kono M, Nakashima MN, Wada M. Determination of donepezil hydrochloride in human and rat plasma, blood and brain microdialysates by HPLC with a short C30 column. J Pharm Biomed Anal. 2006; 41:201–206. DOI:
10.1016/j.jpba.2005.10.017. PMID:
16321494.

7. Ponnayyan Sulochana S, Sharma K, Mullangi R, Sukumaran SK. Review of the validated HPLC and LC-MS/MS methods for determination of drugs used in clinical practice for Alzheimer's disease Bioanalytical methods for quantitation of drugs to treat Alzheimer's. Biomed Chromatogr. 2014; 28:1431–1490. DOI:
10.1002/bmc.3116. PMID:
24515838.
8. Korfmacher WA. Foundation review: Principles and applications of LC-MS in new drug discovery. Drug Discov Today. 2005; 10:1357–1367. DOI:
10.1016/S1359-6446(05)03620-2. PMID:
16253874.

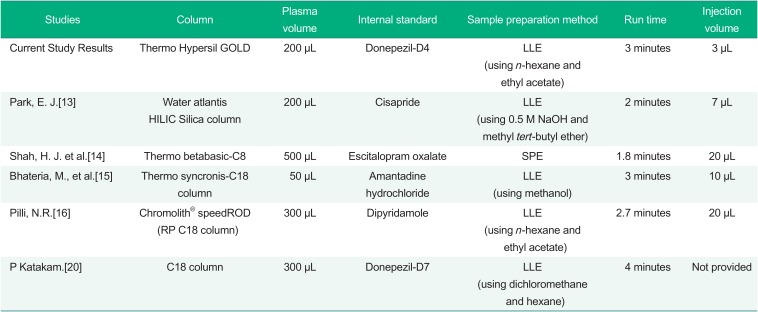
13. Park EJ, Lee HW, Ji HY, Kim HY, Lee MH, Park ES, et al. Hydrophilic interaction chromatography-tandem mass spectrometry of donepezil in human plasma: Application to a pharmacokinetic study of donepezil in volunteers. Arch Pharm Res. 2008; 31:1205–1211. DOI:
10.1007/s12272-001-1290-6. PMID:
18806965.

14. Shah HJ, Kundlik ML, Pandya A, Prajapati S, Subbaiah G, Patel CN, et al. A rapid and specific approach for direct measurement of donepezil concentration in human plasma by LC-MS/MS employing solid-phase extraction. Biomed Chromatogr. 2009; 23:141–151. DOI:
10.1002/bmc.1095. PMID:
18823072.

15. Bhateria M, Ramakrishna R, Pakala DB, Bhatta RS. Development of an LC–MS/MS method for simultaneous determination of memantine and donepezil in rat plasma and its application to pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2015; 1001:131–139. DOI:
10.1016/j.jchromb.2015.07.042.

16. Pilli NR, Inamadugu JK, Kondreddy N, Karra VK, Damaramadugu R, Rao JV. A rapid and sensitive LC-MS/MS method for quantification of donepezil and its active metabolite, 6-o-desmethyl donepezil in human plasma and its pharmacokinetic application. Biomed Chromatogr. 2011; 25:943–951. DOI:
10.1002/bmc.1552. PMID:
21154884.

17. Nováková L, Vlčková H. A review of current trends and advances in modern bio-analytical methods: chromatography and sample preparation. Anal Chim Acta. 2009; 656:8–35. DOI:
10.1016/j.aca.2009.10.004. PMID:
19932811.

18. Pavlovič DM, Babič S, Horvat AJM, Kaštelan-Macan M. Sample preparation in analysis of pharmaceuticals. Trac-Trends Anal Chem. 2007; 26:1062–1075. DOI:
10.1016/j.trac.2007.09.010.
19. Dejaegher B, Mangelings D, Vander Heyden Y. Method development for HILIC assays. J Sep Sci. 2008; 31:1438–1448. DOI:
10.1002/jssc.200700680. PMID:
18461571.

20. Katakam P, Kalakuntla RR, Adiki SK, Chandu BR. Development and validation of a liquid chromatography mass spectrometry method for the determination of donepezil in human plasma. J Pharm Res. 2013; 7:720–726. DOI:
10.1016/j.jopr.2013.08.021.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download