Abstract
Case summary
A 70-year-old male presented with a 5-month history of a right upper eyelid mass. The mass appeared as 1.2 ×1.2 cm on the right upper eyelid. A mass excision was performed under frozen section control. The tumor was completely excised with a safety margin clearance and an upper eyelid reconstruction was performed. Histopathological examination revealed a tumor composed of small atypical cells which showed a high nuclear/cytoplasm ratio, nuclear molding, and increased mitotic activity. Immunohistochemical examination revealed positive reactivity for Ki-67, synaptophysin, CD56, and negative reactivity for chromogranin, cytokeratin 20, and thyroid transcription factor-1.
Go to : 
References
1. Remick SC, Hafez GR, Carbone PP. Extrapulmonary small-cell carcinoma. A review of the literature with emphasis on therapy and outcome. Medicine (Baltimore). 1987; 66:457–71.
2. Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for abdominal tumors in 35,825 cases in the United States. J Clin Oncol. 2008; 26:3063–72.
3. Bajetta E, Catena L, Procopio G, et al. Is the new WHO classi fi abdominal of neuroendocrine tumours useful for selecting an appropriate treatment? Ann Oncol. 2005; 16:1374–80.
4. Solcia EKG, Sobin LH. WHO: Histological Typing of Endocrine Tumors. Berlin/New York: Springer;2000. p. 7–13.
5. Faggiano A, Mansueto G, Ferolla P, et al. Diagnostic and abdominal implications of the World Health Organization classi fi cation of neuroendocrine tumors. J Endocrinol Invest. 2008; 31:216–23.
6. Remick SC, Ruckdeschel JC. Extrapulmonary and pulmonary small-cell carcinoma: tumor biology, therapy, and outcome. Med Pediatr Oncol. 1992; 20:89–99.

8. Silkiss RZ, Green JE, Shetlar DJ. Small cell neuroendocrine abdominal of the eyelid. Ophthal Plast Reconstr Surg. 2008; 24:319–21. discussion 321–2.
9. Yamanouchi D, Oshitari T, Nakamura Y, et al. Primary abdominal carcinoma of ocular adnexa. Case Rep Ophthalmol Med. 2013; 2013:281351.
10. Metz KA, Jacob M, Schmidt U, et al. Merkel cell carcinoma of the eyelid: histological and immunohistochemical features with abdominal respect to differential diagnosis. Graefes Arch Clin Exp Ophthalmol. 1998; 236:561–6.
11. Llombart B, Monteagudo C, López-Guerrero JA, et al. Clinicopathological and immunohistochemical analysis of 20 cases of Merkel cell carcinoma in search of prognostic markers. Histopathology. 2005; 46:622–34.

12. Hanly AJ, Elgart GW, Jorda M, et al. Analysis of thyroid transcription factor-1 and cytokeratin 20 separates merkel cell carcinoma from small cell carcinoma of lung. J Cutan Pathol. 2000; 27:118–20.

14. Dancey AL, Rayatt SS, Soon C, et al. Merkel cell carcinoma: a abdominal of 34 cases and literature review. J Plast Reconstr Aesthet Surg. 2006; 59:1294–9.
15. Muqit MM, Roberts F, Lee WR, Kemp E. Improved survival rates in sebaceous carcinoma of the eyelid. Eye (Lond). 2004; 18:49–53.

16. Jabbour J, Cumming R, Scolyer RA, et al. Merkel cell carcinoma: assessing the effect of wide local excision, lymph node dissection, and radiotherapy on recurrence and survival in early-stage abdominal–results from a review of 82 consecutive cases diagnosed abdominal 1992 and 2004. Ann Surg Oncol. 2007; 14:1943–52.
Go to : 
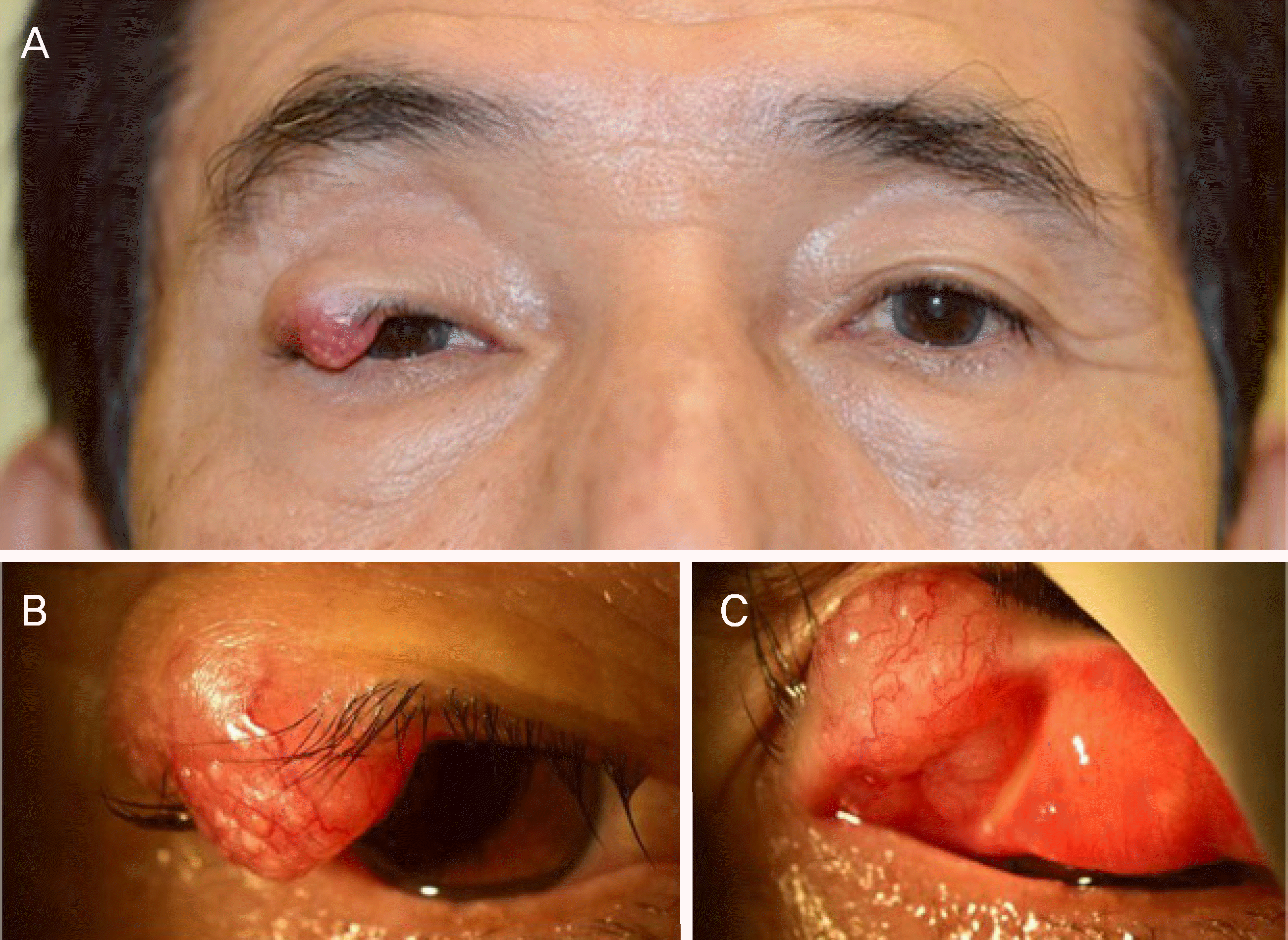 | Figure 1.Clinical photograph of the patient. (A) Clinical photograph demonstrates mass on the lateral side of right upper eyelid. (B) yellowish and papillary mass on the upper eyelid is noted. (C) well vascularized 1.2 × 1.2 cm sized mass on the right upper eyelid is noted. |
 | Figure 2.Clinical photograph of the patient's surgery. (A) Intraoperative photography showing the marked tumor of the upper eyelid. (B) Full-thickness upper eyelid defect is shown after tumor excision under frozen-section control. (C) The tarsoconjunctival flap is made and advanced in the upper eyelid defect. (D) The flap and alloderm graft is sutured to the upper eyelid levator muscle, the medial tarsal remnant, and the lateral canthal tendon remnant. (E) Mobilized orbicularis muscle is shown. (F) A posterior auric-ular skin graft is sutured. |
 | Figure 3.Histopathological examination of mass. (A) Low-power magnification of tumor shows nests of small round cells with increased nuclear to cytoplasmic ratio and hyperchromic nuclei (hematoxylin and eosin, ×100). (B) High-power magnification reveals molding of small round cells with increased nuclear to cytoplasmic ratio (hematoxylin and eosin, ×400). (C-E) Immunohistochemical stains show positivity for synaptophysin, CD56 and negativity for chromogranin, respectively (×100). |
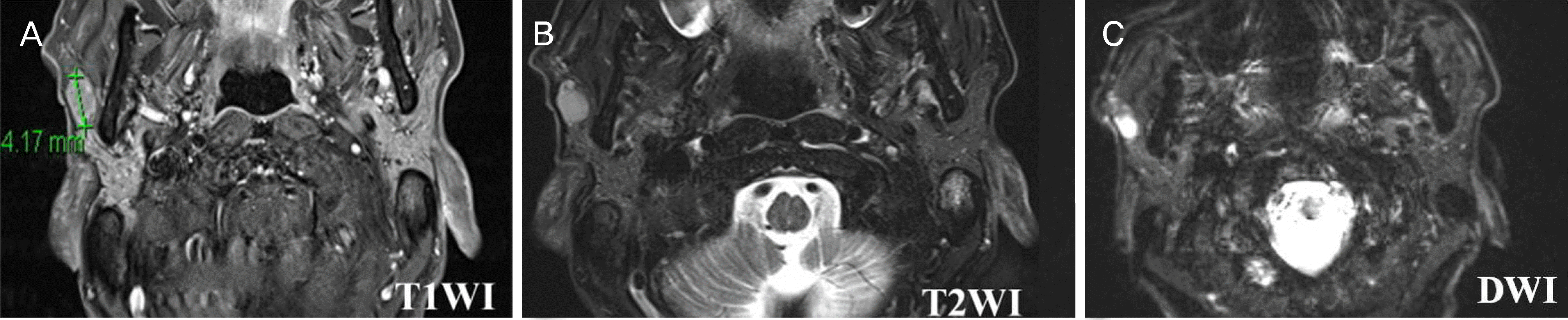 | Figure 4.Head and neck magnetic resonance imaging demonstrating nodule in the right parotid gland. (A) T1-weighted axial image demonstrating 1.5 cm-sized mass on superficial lobe of the right parotid gland with low signal intensity. (B) T2-weighted axial image demonstrating high signal intensity mass on the right parotid gland. (C) Diffusion weighted image demonstrating diffusion restricted 1.5 cm-sized nodule in right parotid gland. T1WI = T1-weighted imaging; T2WI = T2-Weighted imaging; DWI = diffusion weighted imaging. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download