Abstract
Purpose
Methods of intraocular lens (IOL) calculations for cataract surgery after myopic corneal refractive surgery were compared.
Methods
The medical records of 23 eyes from 20 patients were retrospectively analyzed. Five methods – Haigis L and Barrett no history method from Post-Refractive IOL Calculator of American Society of Cataract and Refractive Surgery (ASCRS), Shammas no history method, Haigis suite from IOL Master 700, and clinical history method – were used.
Results
The Barrette no history method from the ASCRS showed the lowest arithmetic errors, while the Haigis-L from the ASCRS showed the lowest absolute prediction errors (−0.38 ± 0.99 diopters [D] and 0.76 ± 0.68 D, respectively). The percentages of refractive prediction errors within 0.50 D and 1.00 D of the Barrett no history method were 52.2% and 82.6%, respectively.
Go to : 
References
1. Abulafia A, Hill WE, Wang L, et al. Intraocular lens power calculation in eyes after laser in situ keratomileusis or photorefractive keratectomy for myopia. Asia-Pac J Ophthalmology. 2017; 6:332–8.
2. Hoffer KJ. Intraocular lens power calculation after previous laser refractive surgery. J Cataract Refract Surg. 2009; 35:759–65.

3. Shammas HJ, Shammas MC. No-history method of intraocular lens power calculation for cataract surgery after myopic laser in situ keratomileusis. J Cataract Refract Surg. 2007; 33:31–6.

4. Potvin R, Hill W. New algorithm for intraocular lens power abdominals after myopic laser in situ keratomileusis based on rotating Scheimpflug camera data. J Cataract Refract Surg. 2015; 41:339–47.
5. Barrett GD. An improved universal theoretical formula for intraocular lens power prediction. J Cataract Refract Surg. 1993; 19:713–20.

6. Patel RH, Karp CL, Yoo SH, et al. Cataract surgery after refractive surgery. Int Ophthalmol Clin. 2016; 56:169–80.

7. Shammas MC, Shammas HJ. Post-LASIK IOL power abdominals: where are we in 2012. Curr Ophthalmol Rep. 2013; 1:39–44.
8. Choi DM, Thompson RW Jr, Price FW Jr. Incisional refractive surgery. Curr Opin Ophthalmol. 2002; 13:237–41.

9. Peyman GA, Larson B, Raichand M, Andrews AH. Modification of rabbit corneal curvature with use of carbon dioxide laser burns. Ophthalmic Surg. 1980; 11:325–9.
10. Kugler LJ, Wang MX. Lasers in refractive surgery: history, abdominal, and future. Appl Opt. 2010; 49:F1–9.
11. Gale RP, Saldana M, Johnston RL, et al. Benchmark standards for refractive outcomes after NHS cataract surgery. Eye (Lond). 2009; 23:149–52.

12. Abulafia A, Hill WE, Koch DD, et al. Accuracy of the Barrett True-K formula for intraocular lens power prediction after laser in situ keratomileusis or photorefractive keratectomy for myopia. J Cataract Refract Surg. 2016; 42:363–9.

13. Olsen T. Sources of error in intraocular lens power calculation. J Cataract Refract Surg. 1992; 18:125–9.

14. Wang JK, Hu CY, Chang SW. Intraocular lens power calculation using the IOLMaster and various formulas in eyes with long axial length. J Cataract Refract Surg. 2008; 34:262–7.

15. Bang S, Edell E. Yu Q, et al. Accuracy of intraocular lens abdominals using the IOLMaster in eyes with long axial length and a comparison of various formulas. Ophthalmology. 2011; 118:503–6.
16. Zaldivar R, Shultz MC, Davidorf JM, Holladay JT. Intraocular lens power calculations in patients with extreme myopia. J Cataract Refract Surg. 2000; 26:668–74.

17. Lege BA, Haigis W. Laser interference biometry versus ultrasound biometry in certain clinical conditions. Graefes Arch Clin Exp Ophthalmol. 2004; 242:8–12.

18. Randleman JB, Hewitt SM, Stulting RD. Refractive changes after posterior segment surgery. Ophthalmol Clin North Am. 2004; 17:521–6. v-vi.

19. Slusher MM, Ford JG, Busbee B. Clinically significant corneal abdominal and pars plana vitrectomy. Ophthalmic Surg Lasers. 2002; 33:5–8.
20. Hayashi H, Hayashi K, Nakao F, Hayashi F. Corneal shape changes after scleral buckling surgery. Ophthalmology. 1997; 104:831–7.

21. Weinberger D, Lichter H, Loya N, et al. Corneal topographic changes after retinal and vitreous surgery. Ophthalmology. 1999; 106:1521–4.

22. Wirbelauer C, Hoerauf H, Roider J, Laqua H. Corneal shape changes after parsplana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 1998; 236:822–8.
Go to : 
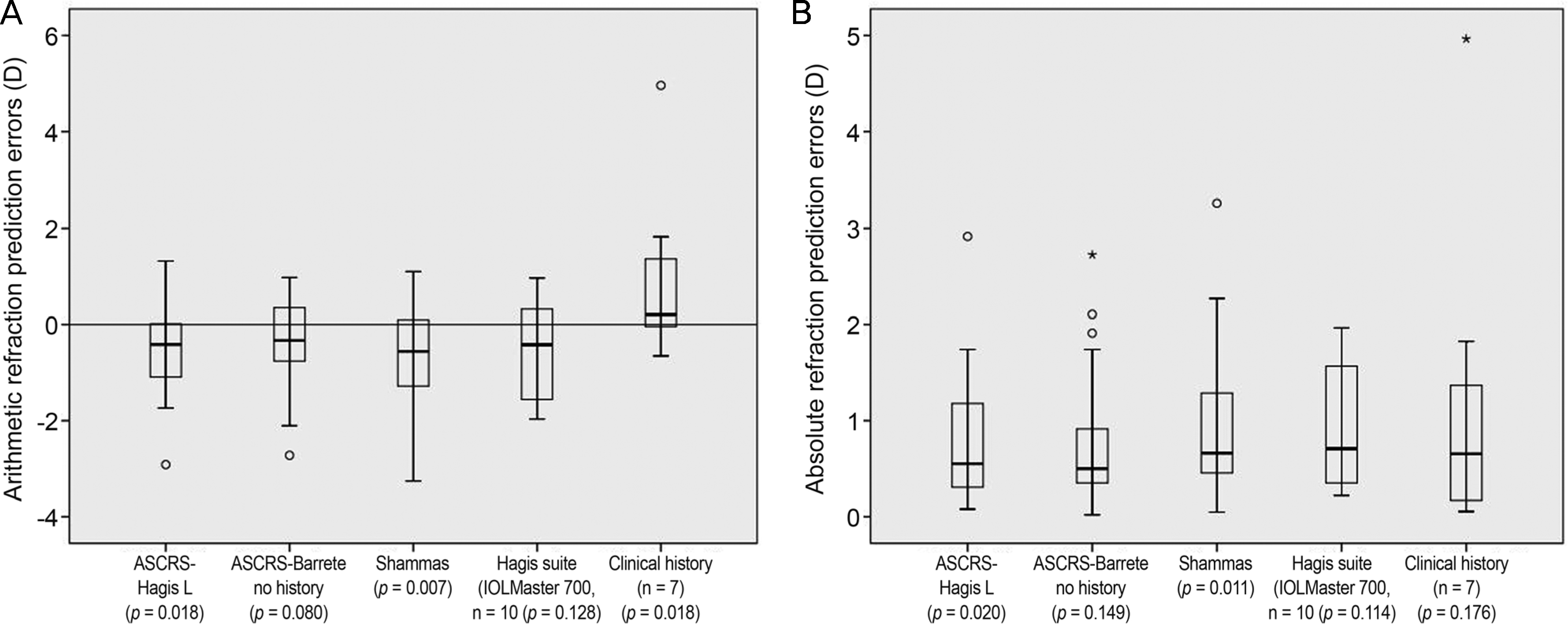 | Figure 1.Box-plots of arithmetic (A) and absolute refraction prediction errors (B) of patients. It shows the distribution of errors of prediction by five methods. (A) 1-sample t test was used to determine whether the arithmetic refraction prediction errors were significantly different from zero and p-values were presented. (B) Wilcox signed rank test was performed to determine the statistical significance for absolute refraction prediction errors and p-values were presented. ASCRS = American Society of Cataract and Refractive Surgery. º = outliers; * = extreme outliers. |
Table 1.
Demographics and characteristics of patients
Table 2.
Mean arithmetic and absolute refraction prediction errors of patients
Table 3.
Percentage of eyes within ±0.25 D, ±0.50 D, ±0.75 D and ±1.00 D from target refraction of subjects




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download