Abstract
Objective
This study was performed to investigate the rate of tooth movement and histological characteristics of extraction sockets those were subjected to corticotomy.
Methods
A split-mouth randomized controlled trial experiment was designed. Thirty-two adult, male Wistar rats were divided into 2 groups: healing extraction socket (H) and recent extraction socket (R); these groups were randomly classified into 4 subgroups (0/7/21/60 days). The first maxillary molar was extracted on 1 side and 2 months were allowed for complete bone healing; then, the corresponding molar was extracted on the other side and surgical intervention was performed at the mid-alveolar point of the first maxillary molar. Ten grams of continuous force was applied. The outcomes measured were rate of tooth movement, percentage of periodontal space and histological evaluation. The rate of tooth movement was calculated as the measured distance divided by the duration of molar movement. Histomorphometric evaluations were performed on the second and third maxillary molars. The Wilcoxon signed rank test was used to compare differences between the two groups.
Corticotomy is a surgical technique that involves the reflection of flaps, followed by buccal and lingual cortical plate penetration with intact spongy bone.1 This technique is based on the concept of stimulating the remodeling process for conventional orthodontic tooth movement, known as the “regional acceleratory phenomenon” (RAP). This process activates temporary physiologic bone healing of injured tissue, buccal and lingual plate, as well as the surrounding surgically treated tissue, bone marrow, and periodontal ligament (PDL) space, thereby causing reduced bone density.23 It is a temporary stage of localized soft- and hard-tissue remodeling, which results in the rebuilding of injured sites to a normal state through recruitment of osteoclasts and osteoblasts by local intercellular mediator mechanisms; these involve precursors, supporting cells, blood capillaries, and lymph.4 Sebaoun et al.5 reported that surgical injury to the rat alveolus induced a three-fold increase in anabolic and catabolic remodeling by the third week after corticotomy; Wang et al.6 indicated that bone resorption production around moving teeth increased for up to 21 days after corticotomy. In the same conditions, Mostafa et al.7 measured a two-fold increase in the rate of tooth movement in dogs, along with an increase in bone turnover and RAP. In another animal study by Iino et al.,8 the third molars mesialized significantly faster than the control side in 12 dogs that underwent corticotomy.
Although there are many histological reports of corticotomy, most concentrate on recent sites of extraction (socket) or non-extraction (bone). The speed of orthodontic tooth movement into an extraction site remains controversial. Compared with a healed site, accelerated tooth movement into a recent site has been reported. Liou and Huang9 reported that tooth movement increased from 1 mm/month to 6.5 mm/3 weeks in a study of canine retraction into a socket generated by recent first premolar extraction. Häsler et al.10 found that, in the activation period, tooth movement was faster at sites of recent extraction. However, the total distance did not differ between the groups. In contrast, Diedrich and Wehrbein11 found greater tooth retraction velocity into a healed socket than into a recent socket. Notably, this was associated with low bone density, mature lamellar bone, and gingival invagination in the healed socket; there was high bone density and less mature lamellar bone in the recent socket. Additionally, in an animal study, Murphey et al.12 observed compression and tension areas of fresh and 6-week healed sockets in monkeys. They concluded that there were more osteoclastic activities at fresh sites and new bone formation at healed sites. This implies greater restrained tooth movement into healed sockets than into recent sockets.
Based on previous evidence, clear understanding of surgically accelerated tooth movement into a different extraction socket has not been achieved. Therefore, this study was undertaken to compare the rate of corticotomy-assisted orthodontic tooth movement of maxillary second molars that were moved toward recent and healed extracted sockets of maxillary first molars; it also aimed to compare the overall histological appearance of the maxillary second molar area at different time periods after the application of orthodontic force.
This study used a split-mouth, randomized controlled trial approach that was approved by the Animal Ethics Committee, Prince of Songkla University. Thirty-two adult male Wistar rats, aged 3 to 4 months and weighing 150 to 250 g were randomly divided into two groups (right or left side) by using a random number table. Group 1 was the healed socket group, while group 2 was the recent socket group. In group 1, the maxillary first molar was extracted and a post-extraction period of at least 2 months was allowed for the socket to completely heal before beginning corticotomy combined with orthodontic tooth movement. In group 2, extraction of the maxillary first molar was performed concurrently with corticotomy combined with orthodontic tooth movement.
After random allocation of the rats into 2 groups as above, the 32 Wistar rats in each group were then randomly divided into 4 subgroups on the basis of the number of days between corticotomy induction and mandible removal. Therefore, there were 8 subgroups, which comprised 4 healed socket subgroups and 4 recent socket subgroups. Each subgroup contained 8 sides (Figure 1).
The surgical procedure was performed while the rats were under anesthesia. The rats were weighed to calculate the dose of anesthetic drug; then, intramuscular injection was performed by 90 mg/kg ketamine hydrochloride (Ketaset III; Zoetis Inc., Parsippany, NJ, USA) and 10 mg/kg xylazine hydrochloride (AnaSed; Lloyd Inc., Shenandoah, IA, USA) at a ratio of 7:3. Subsequently, the maxillary first molar was elevated with a spatula number 7 and gently extracted with artery forceps. Decortication was performed by making a 5-mm incision mesially to the upper second molars, elevating the full-thickness periosteal flap, and decorticating the mid-alveolar ridge at 2 intramedullary points, 0.25 mm in depth, by using a slow-speed handpiece with a small carbide round bur (0.5 mm diameter) (Figures 2 and 3). The coil springs were inserted immediately after corticotomy and flaps were sealed.
The orthodontic force consisted of ligating 0.008 inch ligature wires at the second maxillary molar and incisors. The second maxillary molar protraction forces were generated by using light nickel-titanium closed coil springs (length = 8 mm; diameter = 1.5 mm) (Dentos, Inc., Daegu, Korea), which extended 5 mm to produce 10 g of force.
For the purpose of determining the rate of tooth movement, impressions were taken with light body silicone (Affinis; Coltene/Whaledent Inc., Altstätten, Switzerland) to serve as reference models, prior to the surgical procedure. The rate of tooth movement was calculated by dividing the distance between “the distal surfaces of the second molar to the mesial surfaces of the third molar” by “the number of days of protraction time”. The amount of tooth movement was measured from the study models by using a digital veneer caliper (Mitutoyo; Sumipol Co., Ltd., Bangkok, Thailand).
At the end of each time point, 8 rats from each group were euthanized and the mandibles were removed. The maxilla was fixed in 10% neutral buffered formalin within 48 hours after removal; it was then decalcified with 10% ethylenediaminetetraacetic acid at room temperature for 21 days and dissected into recent and healed sides or half maxilla. Samples were prepared at 5 mm thicknesses and embedded in paraffin blocks. The paraffin-embedded samples were sectioned by orienting the occlusal surface of the second and third maxillary molars toward the paraffin block.
Each sample was cut into 10 sections, from extracted maxillary first molar through maxillary third molar area, which was horizontally parallel to the occlusal plane. Each section was 3 μm thick and separated from the next section by 144 μm because the average mesial rat molar root length is 1,415 ± 430 μm.13 Sections for general histological examination were stained with hematoxylin and eosin and examined for overall morphology, as well as catabolic activity. Overall appearances were recorded to comparatively explain the pressure and tension of maxillary second molar roots in both recent and healed groups, for every time period of the study.
To determine the percentage of PDL space, the measuring program counted the pixels of periodontal space around the mesiopalatal root of the second maxillary molar. The calculated value of periodontal space of the mesiopalatal root of the second molar was performed by CAD-KAS Measure-Pictures version 1.0 (freeware), with the following formula:
Then, pixel area values of the periodontal space were transformed into percentages of (PDL)/(total PDL + root area) to compare between and within the healed and recent groups at each time point.
Normality was tested with the Shapiro–Wilk test to examine the distribution of data. Since the data were not normally distributed, nonparametric tests were used. To compare differences between the healed and recent socket groups, the Wilcoxon signed-rank test was used. Kruskal–Wallis ANOVA was used to compare durations after orthodontic force application, between the recent and healed groups. Significant differences were defined as α < 0.05. Measurements of all samples were repeated twice and intraobserver reliability was assessed by using the intraclass correlation coefficient.
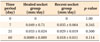
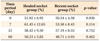
In the pretreatment phase, the mean distances between the distal surface of the second molar and the mesial surface of the third molar were not significantly different between the groups. After the surgical and orthodontic procedures, the distances of tooth movement in each group were measured and the rates of tooth movement were calculated. In the post-treatment phase, tooth movement velocities at the recent side at 0, 7, 21, and 60 days after corticotomy were not significantly faster than at the healed side (Table 1).
Histological analysis showed that at day 0, normal alveolar bone architecture, with intact lamina dura and PDL space, was present in both recent and healed groups. On day 7, the interradicular bone had decreased, while the nonmineralized tissue and PDL space had increased. Viable periodontal cells and multinucleated osteoclast-like cells could be detected in some areas on the margin of the bundle bone, adjacent to the compressed PDL. This was indicative of direct bone resorption. At day 21, the interradicular bone was mostly replaced by nonmineralized tissue that was continuous with the PDL space. Fewer countable cells were observed, compared with day 7. Finally, on day 60, the nonmineralized tissues were replaced with more newly formed bone around the interradicular area; the periodontal space had also decreased, compared with days 7 and 21. There was a histological difference between healed and recent extraction sites within groups, but not across groups, at all time points (Figure 4). However, the overall second molar areas still comprised less mineralized tissue than at day 0.
Comparisons of the different durations of tooth movement between healed and recent groups, performed to determine the percentages of periodontal space, showed statistically significant differences among time points. Although the width of the periodontal space was significantly different among time points, socket type did not affect the results. Comparisons between healed and recent groups in the same time period indicated no significant differences at each time point (Table 2).
To determine intraobserver reliability, a one-way random effects model of intraclass correlation coefficient was used. In the clinical portion of the study, all samples were re-measured. The average intraclass correlation of the rate of tooth movement was 0.955, which indicated great reliability. In the histological portion of the study, 20% of the samples were used to test the reliability of the percentage of periodontal space calculations. The intraclass correlation of the histological analysis was 0.916, which also indicated great reliability.
Selective alveolar decortication induces a localized increase in turnover of alveolar spongiosa, which increases the rate of tooth movement. However, there are no reports of decortication in recent sockets, compared with healed sockets. Most reports have focused on corticotomy at an edentulous area or recently extracted socket. Therefore, this study focused on a comparison between corticotomy assisted-tooth movement rates, based on different socket types.
In the pretreatment phase, the distances between the upper second and third molars were not significantly different, which indicated that the preliminary data between the two groups were similar. Moreover, the upper second molar movement into the upper first molar extraction sites in the healed socket group (before alveolar decortication) might have impacted the measurement of the tooth movement distances. Fortunately, the timing of tooth extraction did not affect the distance of tooth movement, enabling an accurate comparison to be performed.
In the post-treatment phase, the rates of tooth movement in each time period between recent and healed groups were not different in either short or long periods of tooth movement. This finding implied that corticotomy in the recent socket sites did not accelerate tooth movement more than corticotomy in the healed sites.
Histological appearance in each time period was compared with prior studies of the healing process of molar extraction sockets in rats.14 According to previous studies, blood clotting and granulation tissue formation phase are observed during the first five days after tooth extraction. In the next 5 to 20 days, bone formation occurs. Finally, bone remodeling extends until complete bone healing has occurred, which is 20 to 60 days after extraction.14 Therefore, in this study, the time points of 7, 21, and 60 days were chosen to represent each healing stage. Day 7 was the time of peak bone resorption, while day 21 was the time of maximum bone formation. Finally, at day 60, complete bone remodeling was observed.
Many investigators567 have reported that the decorticated alveolar response of the maxillary cortical plate presented significantly less calcified spongiosa bone surface, greater PDL surface, greater number of osteoclasts, and greater lamina dura apposition width, compared with the control group. These data indicate that cortical activation increases the activity of numerous cellular components, including formative and resorptive cells.
When timing-dependent histological changes were considered, both descriptive and quantitative results of this study were similar to the results of decorticated sites in previous studies.15 The gradual change from normal PDL and bundle bone architecture at day 0, toward the resorptive area and gathering of multiple cells, occurred at day 7. This inflammatory process progressed through day 21; eventually, primary bones were formed in the interradicular areas at day 60.
For the results of an animal study to be useful in clinical applications, a primary concern is whether the findings can represent human physiology. Rat alveolar bone is generally denser than in the human. It shows no osteon, the bone plates lack marrow spaces, and there is a lower amount of alveolar bone surface.16 Although the response of rat tissue to orthodontic tooth movement and the subsequent healing process appear to be three times faster than those same processes in human, the general mechanism remains similar.17
In a case of malocclusion, such as anterior tooth protrusion or crowding, extraction is needed to gain space. The appropriate time for extraction is a factor to consider in the treatment plan. For example, in the case of crowding that requires an extracted space, the timing of the extraction of the first premolar must be considered, either before or after the leveling phase. Therefore, the socket wounds are different between recent and healed extraction sites. In the healed socket, alveolar ridge atrophy tends to occur, which obstructs the tooth movement passing through this defect. Conversely, delaying extraction until after the leveling phase may cause anterior teeth proclination, which would be an esthetically unsatisfactory result and an undesired direction of tooth movement.
From our study results, the timing of extraction may not be a factor that affects the rate of tooth movement, when combined with alveolar bone decortication. Consequently, the extraction and decortication time would depend primarily on the treatment sequence that is most effective for each patient.
• Corticotomy-assisted tooth movement can stimulate tooth movement into healed and recent extraction sites at the same rate.
• Histological analysis of tooth movement in this study revealed regional acceleratory phenomena in each time period of tooth movement, but there were no differences between recent and healed extraction sites.
• The amount of nonmineralized periodontal space was not dependent on the type of socket but was based on the time period of tooth movement during the process of bone healing.
Figures and Tables
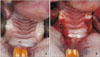 | Figure 2Clinical photographs. A, Complete healing of the healed side at 2 months after extraction. B, After extraction at the recent side and decortication on both sides. |
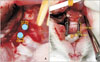 | Figure 3Decortication and orthodontic protocol. A, Midalveolar two-point decortication (diameter, 0.5 mm) at mesial to second maxillary molar. B, Closed coil spring (length, 8 mm; diameter, 1.5 mm) insertion to mesialize the maxillary second molar. |
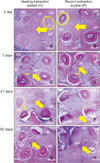 | Figure 4Representative images of histological appearance at the level of the apical third of the maxillary second molar. A, C, E, and G, Healing extraction socket at days 0, 7, 21, and 60, respectively. B, D, F, and H, Recent extraction socket at days 0, 7, 21, and 60, respectively. A and B (Day 0), Full-filled interradicular bone (arrow), continuous and well-defined periodontal space (circle). C and D (Day 7), Non-mineralized tissue and periodontal space (single-ended arrow) was expanded, while the interradicular bone (double-ended arrow) was reduced. E and F (Day 21), Nearly interradicular bone (single-ended arrow) was replaced with non-mineralized tissue, continuous with the periodontal space (double-ended arrow). G and H (Day 60), Non-mineralized tissue was partially replaced with rebuilt interradicular bone (single-ended arrow), while the periodontal space was reduced.MB, Mesiobuccal root; IB, intermediate buccal root; DB, distobuccal root; DP, distopalatal root; MP, mesiopalatal root. Hematoxylin and eosin stain.
|
ACKNOWLEDGEMENTS
This study was supported by a faculty research grant of Prince of Songkla University. The authors would also like to show our gratitude to the Associate Professor Udom Thongudomporn for his comments on an earlier version of the manuscript.
Notes
References
1. Kole H. Surgical operations on the alveolar ridge to correct occlusal abnormalities. Oral Surg Oral Med Oral Pathol. 1959; 12:515–529. concl.

2. Frost HM. The biology of fracture healing. An overview for clinicians Part II. Clin Orthop Relat Res. 1989; (248):294–309.
3. Frost HM. The biology of fracture healing. An overview for clinicians Part I. Clin Orthop Relat Res. 1989; (248):283–293.
4. Yaffe A, Fine N, Binderman I. Regional accelerated phenomenon in the mandible following mucoperiosteal flap surgery. J Periodontol. 1994; 65:79–83.

5. Sebaoun JD, Kantarci A, Turner JW, Carvalho RS, Van Dyke TE, Ferguson DJ. Modeling of trabecular bone and lamina dura following selective alveolar decortication in rats. J Periodontol. 2008; 79:1679–1688.

6. Wang L, Lee W, Lei DL, Liu YP, Yamashita DD, Yen SL. Tisssue responses in corticotomy- and osteotomy-assisted tooth movements in rats: histology and immunostaining. Am J Orthod Dentofacial Orthop. 2009; 136:770.e1–770.e11. discussion 770-1.

7. Mostafa YA, Mohamed Salah Fayed M, Mehanni S, ElBokle NN, Heider AM. Comparison of corticotomy-facilitated vs standard tooth-movement techniques in dogs with miniscrews as anchor units. Am J Orthod Dentofacial Orthop. 2009; 136:570–577.

8. Iino S, Sakoda S, Ito G, Nishimori T, Ikeda T, Miyawaki S. Acceleration of orthodontic tooth movement by alveolar corticotomy in the dog. Am J Orthod Dentofacial Orthop. 2007; 131:448.e1–448.e8.

9. Liou EJ, Huang CS. Rapid canine retraction through distraction of the periodontal ligament. Am J Orthod Dentofacial Orthop. 1998; 114:372–382.

10. Häsler R, Schmid G, Ingervall B, Gebauer U. A clinical comparison of the rate of maxillary canine retraction into healed and recent extraction sites--a pilot study. Eur J Orthod. 1997; 19:711–719.

11. Diedrich P, Wehrbein H. Orthodontic retraction into recent and healed extraction sites. A histologic study. J Orofac Orthop. 1997; 58:90–99.
12. Murphey WH Jr. Oxytetracycline microfluorescent comparison of orthodontic retraction into recent and healed extraction sites. Am J Orthod. 1970; 58:215–239.

13. Hsieh YD, Devlin H, Roberts C. Early alveolar ridge osteogenesis following tooth extraction in the rat. Arch Oral Biol. 1994; 39:425–428.

14. Astrand P, Carlsson GE. Changes in the alveolar process after extractions in the white rat. A histologic and fluorescence microscopic study. Acta Odontol Scand. 1969; 27:113–127.

15. Kim SJ, Park YG, Kang SG. Effects of corticision on paradental remodeling in orthodontic treatment. Angle Orthod. 2009; 79:284–291.
16. Reitan K, Kvam E. Comparative behavior of human and animal tissue during experimental tooth movement. Angle Orthod. 1971; 41:1–14.




 ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download