Abstract
Objective
To date, only a few studies have investigated the relationships between genetic polymorphisms and external apical root resorption (EARR). Hence, the aim of this systematic review was to explore the relationship between different gene polymorphisms and their association with EARR.
Methods
A complete literature search was conducted by two independent reviewers. The PubMed, Science Direct, and Scopus databases were searched. In addition, the bibliographies of all textbooks and relevant articles were searched manually. A meta-analysis was performed using data entered into the electronic databases until February 28, 2017.
Results
On the basis of the search, we identified 17 and 7 publications for the systematic review and meta-analysis, respectively. Odds ratio (OR) was used to evaluate the association of the interleukin 1B (+3954) polymorphism and the risk of EARR. The overall OR from the studies was used to estimate the risk of EARR. However, no association was found and no publication bias was apparent for the risk of EARR in patients receiving orthodontic treatment.
External apical root resorption (EARR) is an adverse effect of orthodontic treatment in most of the patients, which results in the permanent loss of tooth structure from the root apex. Not only due to orthodontic treatment, the incidence of functional root resorption can also be found at the age of 32 to 50 years.1 Few studies have documented extreme resorption sequelae that might negatively affect the patients.
Root resorption is considered an important adjunct effect of orthodontic treatment, which may start in any stage of treatment. Root resorption is usually initiated between the second and fifth weeks of orthodontic treatment, but it can only be identified on radiographs after 3 to 4 months.2 Although many characteristics of root resorption remain unclear, it is considered a complicated biological process, which occurs when forces generated at the root apex surpass the resistance and reparative capability of the periapical tissues. The progress and severity of EARR are exaggerated by various risk factors.3
In 1997, Harris et al.4 conducted the first study on the genetic influence on EARR. Thereafter, various genetic association studies have been conducted on the interrelationship between EARR and orthodontic treatment.5678910
A few studies documented the association of different genes, such as interleukin 1 (IL-1A, IL-1B, and IL-RN), vitamin D receptor, tumor necrosis factor-α, TNSALP, TNFRSF11A, and osteopontin, with EARR during orthodontic treatment.5610 Moreover, various studies have aimed to investigate the association between gene polymorphisms and the risk of EARR during orthodontic treatment. However, the outcomes of these studies have been contradictory.
Therefore, the aim of this systematic review and meta-analysis was to determine whether these gene polymorphisms are associated with EARR during orthodontic treatment.
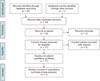
This systematic review adhered to the PRISMA guidelines (Figure 1). Moreover, to better outline the study, the participants, interventions, comparisons, outcomes, and study designs (PICOS) format was followed.11 Literature search was conducted on the PubMed, Science Direct, and Scopus databases (studies included until February 28, 2017) by using the following key words: “Gene, Polymorphism, EARR, external apical root resorption, and orthodontic treatment.” No restrictions were imposed on population, language, sample size, and treatment duration. All suitable articles were selected and their references were rechecked for any relevant articles overlooked during the electronic searches.
The inclusion criteria for the published articles were as follows: 1) association between genetic polymorphisms and the risk of EARR and 2) genotypes determined by calculating the odds ratios (ORs). Moreover, 1) studies with duplication of data, 2) review articles, and 3) studies with insufficient data were excluded. Two reviewers independently reviewed all the articles and selected the studies on the basis of the inclusion and exclusion criteria. No disagreement occurred between the two reviewers. The studies were selected for the systematic review when they fulfilled the appropriate criteria.
For quality evaluation, the strengthening the reporting of genetic association (STREGA) statement and checklist were followed.12 Methodologic quality scores were calculated and ranged from 0 (lowest) to 20 (highest). Studies with scores < 10 were considered low-quality studies and those with scores ≥ 10 were considered high-quality studies.
A meta-analysis was performed on the basis of the association of a similar gene (IL-1B [+3954]) with the EARR outcomes of selected studies.
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 22.0 (IBM Co., Armonk, NY, USA) and Comprehensive Meta-Analysis (CMA) Software version 3 (Biostat, Englewood, NJ, USA). To assess the interobserver agreement of the grading qualities of the studies, Cohen's kappa analysis was performed to identify the quality of the studies. The level of agreement was graded according to the kappa scores, with 0.81–1, 0.61–0.80, 0.41–0.60, 0.21–0.40, and < 0.20 indicating near perfect, substantial, moderate, fair, and poor agreement, respectively.13
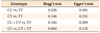
ORs with corresponding 95% confidence intervals were used to estimate the power of the association between EARR and gene polymorphisms. A dominant model (CC + CT vs. TT), co-dominant model (CC vs. TT or CT vs. TT), and recessive model (CC vs. CT + TT) were used to obtain the pooled OR. Residuals were checked for suspected outliers in each study. Heterogeneity was tested using Q statistics and I2 statistics. I2 < 50% and a p-value of Q statistics > 0.10 indicated a lack of heterogeneity across the studies.
Publication bias was estimated using the funnel plot, wherein an asymmetric plot indicated possible bias. Begg's14 and Egger's test15 methods were used for assessing the funnel plot. A p-value < 0.05 was considered to indicate the presence of bias among the studies, and a p-value > 0.05 was considered to indicate the opposite.
The PRISMA guidelines were strictly followed to select the articles as presented in Figure 1. The total number of articles identified for this study was 40: 14 from PubMed, 14 from Scopus, and 12 from Science Direct, after being confirmed by the two independent reviewers. One article was included after manually searching the bibliographies of all the relevant articles. After removing duplicates, 19 publications were selected for the current study. Two studies were further excluded as they did not meet the inclusion criteria. After excluding the duplicates and including the manually searched article, a total of 17 articles were finally included in the systematic review (Table 1) and 7 were included in the meta-analysis (Table 2).
Quality assessment of the selected studies for this systematic review according to the STREGA guidelines1415 was performed by two independent reviewers. Among the 17 selected studies, 11 were classified as high-quality studies (≥ 10) and 6 as low-quality studies (< 10) (Table 3). Inter-rater reliability for these five criteria was 0.728, which represented a substantial level of agreement. Cohen's kappa score for most of the criteria showed the absolute level of inter-rater agreement (Table 4).
The analysis of the association of EARR risk with the IL-1B (+3954) polymorphism showed that none of the dominant, co-dominant, and recessive models were associated with the risk of EARR. Moreover, although a less-expected frequency residual was detected because of a sampling error, it could still be included in the meta-analysis in the random-effect model without heavily influencing the outcome (Figures 2, 3, 4, 5 and Table 5).
Both clinical and statistical heterogeneity were observed among the studies in the dominant, co-dominant, and recessive models (Table 6). Sensitivity analysis was performed after omitting one study at each step to observe the influence of each study on the pooled OR. However, the heterogeneity persisted irrespective of which study was omitted.
In this systematic review, 11 of the 17 studies were classified as high-quality studies and 6 as low-quality studies. Many studies suggested that genetic polymorphisms may be associated with EARR.681617181920212223 However, few studies opposed this association.71024 The STREGA guidelines were formulated for conducting geneticassociation studies to ensure they could attain a score of 20 regardless of the outcome.12 However, in the current systematic review, none of the 11 studies classified as high-quality studies (score ≥ 10) could attain a score of 20. In contrast, the low-quality studies (score < 10) failed to fulfill the minimum categories of the STREGA guidelines, which might lead to a biased outcome in genetic-association studies. Most of the genetic studies associated with orthodontic treatment rarely comply with the STREGA or any other genetic guidelines. Therefore, specific studies may introduce bias owing to methodological inaccuracies.
A previous study revealed that under the same mechanical stress during orthodontic treatment, many subjects showed a low resistance to EARR, whereas others experienced severe EARR.4 In this respect, many researchers investigated a possible link between polymorphic targets in different genes and the association of EARR with orthodontic treatment.789 The current systematic review showed that 15 of the 17 studies aimed to determine the association of IL-1 cluster genes with EARR.
Cytokines have been revealed to disturb bone remodeling and are presently assumed to play a vigorous role in both physiological and pathological bone regulation. Many cells are involved in bone resorption and formation, including osteoclasts, osteoblasts, and bone marrow cells.2526 IL-1 is one of the most important monocyte-macrophage-derived cytokines that acts as a potent stimulator of bone resorption in organ culture.27 IL-1 has been verified to stimulate bone resorption both in vitro and in vivo.
At present, the IL-1B (+3954) gene polymorphism has been documented as a high-risk factor associated with EARR.5 Patients who are homozygous for the IL-1B (+3954) allele 1 have a 95% probability of developing root resorption of more than 2 mm.28 Among the 17 articles selected for this systematic review, 14 were conducted to determine the association between IL-1 and IL-1B (+3954) polymorphisms and EARR. Owing to their similar methodology, 7 studies were selected for this meta-analysis to determine the relationship between the IL-1B (+3954) gene polymorphism and EARR. The pooled result showed a negative association between EARR and the IL-1B (+3954) gene polymorphism. A small sample size, inclusion of different ethnic groups, and other confounding factors might be the reasons behind this insignificant association. The studies included in this meta-analysis had sample sizes less than 100, except for 2 studies. Moreover, 5 of 7 studies were conducted in different ethnic groups, and the frequency of genotypes varied among ethnicities. In addition, confounding factors such as sex, treatment modalities, duration of treatment, smoking, diabetes, and body mass index may influence the occurrence of EARR, but these were not considered in this study; therefore, the outcome may be inaccurate. In addition, different radiography techniques, such as lateral cephalography, orthopantomography, and cone beam computed tomography, were used for measuring the EARR in different studies. The different precisions of these different radiography techniques may affect the outcome of the analyses. Iglesias-Linares et al.8 conducted three different studies using IL-1B (+3954) and EARR aiming to obtain different outcomes. Although the ethnic groups, genotyping methods, and biological markers were identical in the aforementioned studies, the sample sizes and objectives were different, and this resulted in the different outcomes.82223
Although only some studies found a positive association between IL-1B gene polymorphisms and EARR, the negative result of this meta-analysis will be a matter of argument. Therefore, we conducted statistical heterogeneity tests by using I2 and Q statistics, which indicated that the significant heterogeneity observed in the current meta-analysis may alternatively suggest a bias in the design of some of the analyzed studies.29 Nevertheless, clinical heterogeneity is always present in a metaanalysis and is based on the sample size, study design, intervention, and outcomes. The current meta-analysis also represented clinical heterogeneity based on the ethnic background, sample size, genotyping methods, and outcomes.
EARR is a common phenomenon after orthodontic treatment.233031 Therefore, assessing the risk of developing EARR after orthodontic treatment is an important factor for successful treatment. Progression of EARR is a multifactorial event. The results of the current systematic review and meta-analysis may, together with further research, support that several genes are closely associated with EARR. Monitoring and screening for EARR risk in patients receiving orthodontic treatment are valuable components for ensuring more effective control and prevention of EARR.
· The outcome of this systematic review shows that different gene polymorphisms may indicate the occurrence of EARR in some individuals undergoing orthodontic treatment.
· The present study also proposes certain recommendations for prospective researchers assessing gene polymorphisms in patients receiving orthodontic treatment.
· The meta-analysis suggested that the IL-1B polymorphism is not associated with a predisposition to EARR, but there was significant heterogeneity among the study results. However, the heterogeneity among the published studies remains inexplicable.
· Although IL-1B is considered a promising gene for predicting EARR in patients receiving orthodontic treatment, further better-controlled studies are warranted to verify this association.
Figures and Tables
Figure 2
Forest plots of external apical root resorption risk associated with the interleukin-1B polymorphism (CC vs. TT). OR, Odds ratio; 95% CI, 95% confidence interval.

Figure 3
Forest plots of external apical root resorption risk associated with the interleukin-1B polymorphism (CT vs. TT). OR, Odds ratio; 95% CI, 95% confidence interval.

Figure 4
Forest plots of external apical root resorption risk associated with the interleukin-1B polymorphism (CC+CT vs. TT). OR, Odds ratio; 95% CI, 95% confidence interval.

Figure 5
Forest plots of external apical root resorption risk associated with the interleukin-1B polymorphism (CC vs. CT+TT). OR, Odds ratio; 95% CI, 95% confidence interval.

ACKNOWLEDGEMENTS
The authors are grateful for the financial support from Universiti Sains Malaysia (Graduate Assistant Scheme to SAN and Vice Chancellor Award to FS).
Notes
References
1. Wehrbein H, Fuhrmann RA, Diedrich PR. Human histologic tissue response after long-term orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1995; 107:360–371.

2. Parker RJ, Harris EF. Directions of orthodontic tooth movements associated with external apical root resorption of the maxillary central incisor. Am J Orthod Dentofacial Orthop. 1998; 114:677–683.

3. Brezniak N, Wasserstein A. Orthodontically induced inflammatory root resorption. Part I: the basic science aspects. Angle Orthod. 2002; 72:175–179.
4. Harris EF, Kineret SE, Tolley EA. A heritable component for external apical root resorption in patients treated orthodontically. Am J Orthod Dentofacial Orthop. 1997; 111:301–309.

5. Al-Qawasmi RA, Hartsfield JK Jr, Everett ET, Flury L, Liu L, Foroud TM, et al. Genetic predisposition to external apical root resorption. Am J Orthod Dentofacial Orthop. 2003; 123:242–252.

6. Fontana ML, de Souza CM, Bernardino JF, Hoette F, Hoette ML, Thum L, et al. Association analysis of clinical aspects and vitamin D receptor gene polymorphism with external apical root resorption in orthodontic patients. Am J Orthod Dentofacial Orthop. 2012; 142:339–347.

7. Gülden N, Eggermann T, Zerres K, Beer M, Meinelt A, Diedrich P. Interleukin-1 polymorphisms in relation to external apical root resorption (EARR). J Orofac Orthop. 2009; 70:20–38.

8. Iglesias-Linares A, Yañez-Vico RM, Ballesta S, Ortiz-Ariza E, Mendoza-Mendoza A, Perea E, et al. Interleukin 1 gene cluster SNPs (rs1800587, rs1143634) influences post-orthodontic root resorption in endodontic and their contralateral vital control teeth differently. Int Endod J. 2012; 45:1018–1026.

9. Iglesias-Linares A, Yañez-Vico RM, Moreno-Fernández AM, Mendoza-Mendoza A, Orce-Romero A, Solano-Reina E. Osteopontin gene SNPs (rs9138, rs11730582) mediate susceptibility to external root resorption in orthodontic patients. Oral Dis. 2014; 20:307–312.

10. Tomoyasu Y, Yamaguchi T, Tajima A, Inoue I, Maki K. External apical root resorption and the interleukin-1B gene polymorphism in the Japanese population. Orthod Waves. 2009; 68:152–157.

11. Roscoe MG, Meira JB, Cattaneo PM. Association of orthodontic force system and root resorption: A systematic review. Am J Orthod Dentofacial Orthop. 2015; 147:610–626.

12. Aminoshariae A, Aminoshariae A, Valiathan M, Kulild JC. Association of genetic polymorphism and external apical root resorption. Angle Orthod. 2016; 86:1042–1049.

13. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33:159–174.

14. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50:1088–1101.

15. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–634.

16. Guo Y, He S, Gu T, Liu Y, Chen S. Genetic and clinical risk factors of root resorption associated with orthodontic treatment. Am J Orthod Dentofacial Orthop. 2016; 150:283–289.

17. Bastos Lages EM, Drummond AF, Pretti H, Costa FO, Lages EJ, Gontijo AI, et al. Association of functional gene polymorphism IL-1beta in patients with external apical root resorption. Am J Orthod Dentofacial Orthop. 2009; 136:542–546.

18. Iglesias-Linares A, Yañez-Vico RM, Ortiz-Ariza E, Ballesta S, Mendoza-Mendoza A, Perea E, et al. Postorthodontic external root resorption in root-filled teeth is influenced by interleukin-1β polymorphism. J Endod. 2012; 38:283–287.

19. Iglesias-Linares A, Yañez-Vico R, Ballesta-Mudarra S, Ortiz-Ariza E, Ortega-Rivera H, Mendoza-Mendoza A, et al. Postorthodontic external root resorption is associated with IL1 receptor antagonist gene variations. Oral Dis. 2012; 18:198–205.

20. Iglesias-Linares A, Yañez-Vico RM, Ballesta-Mudarra S, Ortiz-Ariza E, Mendoza-Mendoza A, Perea-Pérez E, et al. Interleukin 1 receptor antagonist (IL1RN) genetic variations condition post-orthodontic external root resorption in endodontically-treated teeth. Histol Histopathol. 2013; 28:767–773.
21. Pereira S, Lavado N, Nogueira L, Lopez M, Abreu J, Silva H. Polymorphisms of genes encoding P2X7R, IL-1B, OPG and RANK in orthodontic-induced apical root resorption. Oral Dis. 2014; 20:659–667.

22. Pereira S, Nogueira L, Canova F, Lopez M, Silva HC. IRAK1 variant is protective for orthodontic-induced external apical root resorption. Oral Dis. 2016; 22:658–664.

23. Sharab LY, Morford LA, Dempsey J, Falcão-Alencar G, Mason A, Jacobson E, et al. Genetic and treatmentrelated risk factors associated with external apical root resorption (EARR) concurrent with orthodontia. Orthod Craniofac Res. 2015; 18:Suppl 1. 71–82.

24. Linhartova P, Cernochova P, Izakovicova Holla L. IL1 gene polymorphisms in relation to external apical root resorption concurrent with orthodontia. Oral Dis. 2013; 19:262–270.

25. Manolagas SC, Jilka RL. Cytokines, hematopoiesis, osteoclastogenesis, and estrogens. Calcif Tissue Int. 1992; 50:199–202.

26. Roodman GD. Role of cytokines in the regulation of bone resorption. Calcif Tissue Int. 1993; 53:Suppl 1. S94–S98.

27. Gowen M, Wood DD, Ihrie EJ, McGuire MK, Russell RG. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983; 306:378–380.

28. Segal GR, Schiffman PH, Tuncay OC. Meta analysis of the treatment-related factors of external apical root resorption. Orthod Craniofac Res. 2004; 7:71–78.

29. Pan Z, Trikalinos TA, Kavvoura FK, Lau J, Ioannidis JP. Local literature bias in genetic epidemiology: an empirical evaluation of the Chinese literature. PLoS Med. 2005; 2:e334.





 ePub
ePub Citation
Citation Print
Print










 XML Download
XML Download