Abstract
Background
Transfusion-transmissible hepatitis B virus (HBV) infection is a major problem worldwide. Recently, confirmatory nucleic acid tests (NATs) for HBV DNA have been employed in several countries. We assessed the prevalence and yearly trends of HBV infection in blood donors in the Eastern Province of Saudi Arabia, screening for HBV surface antigen (HBsAg), antibody against HBV core antigen (anti-HBc), and HBV DNA.
Methods
Between 2011 and 2015, a total of 22,842 donors were screenedfor HBsAg, anti-HBc, and HBV DNA using the HBsAg Qualitative II kit (Abbott, Ireland Diagnostics Division, Sligo, Ireland), ARCHITECT Anti-hepatitis B core antigen antibody (HBc) II Assay kit (Abbott GmbH & Co. KG, Wiesbaden, Germany), and NAT Procleix Ultrio Elite Assay kit (Grifols Diagnostic Solutions Inc., Los Angeles, CA, USA), respectively.
Results
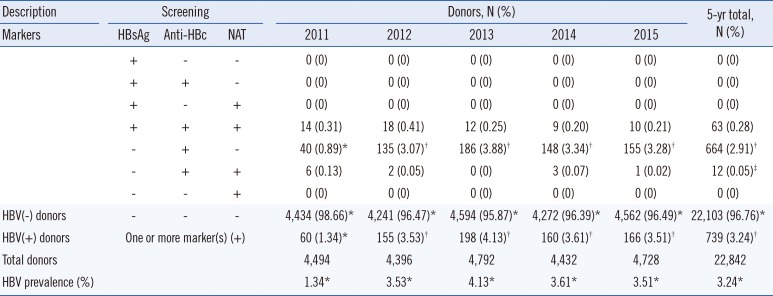
A total of 739 (3.24%) donors were HbsAg(+), anti-HBc(+), or HBV DNA(+); 63 (0.28%) were HbsAg(+), anti-HBc(+), and HBV DNA(+). Twelve (0.05%) were anti-HBc(+) and HBV DNA(+) but HBsAg(−); they were considered to have occult infection. Further, 664 (2.91%) were HBsAg(−) but anti-HBc(+), indicating chronic or resolving infection. HBV prevalence increased significantly from 2011 to 2012, increased marginally till 2013, and showed a decreasing trend from 2013 (P>0.05).
Conclusions
The five-year prevalence of HBV infection among blood donors in the Eastern Province of Saudi Arabia (3.24%) is lower than that reported for other regions in the country. The occult HBV infection rate of 0.05% emphasizes the importance of NATs in isolating potential infectious blood units.
Hepatitis B virus (HBV) infection caused nearly a million (~887,000) deaths worldwide in 2015 [1]. Globally, more than 257 million people live with HBV infection [2]. HBV infection could lead to acute and chronic hepatitis, cirrhosis, and hepatocellular carcinoma, posing a tremendous public health burden [3]. According to the US Centers for Disease Control and Prevention [3], approximately 3,000 people in the US alone and more than 600,000 people worldwide die from hepatitis B-related liver disease each year. Blood transfusion-related HBV infection remains a major concern in transfusion practice. The high rate of the residual risk of transfusion-transmissible HBV reflects the global epidemiology of the virus. Considering the importance of the prevention of HBV transmission through blood transfusion, comprehensive HBV screening programs of blood donors have been implemented worldwide since the early 1970s [4].
A common and essential screening protocol for blood donor samples is serological detection of the marker HBV surface antigen (HBsAg), which is abundantly produced during active infection. However, there still exists a possibility of HBV transmission during transfusion from HBsAg(−) donors, since the absence of the antigen does not completely exclude the presence of the virus. During the window period of the infection (early acute phase or late chronic phase) or in the case of occult infections where viral antigens are not detectable, the HBsAg serological test may give a “non-reactive” result. Accordingly, an additional seromarker, antibody against HBV core antigen (anti-HBc), is tested [5]. Serological reactivity to anti-HBc can indicate active HBV exposure, chronic infection, or resolving infection.
Some advanced laboratories test for HBV DNA in addition to the above seromarkers, using more sensitive, robust, and specific nucleic acid tests (NATs) [6]. Specific amplification and probe hybridization-based NATs are capable of detecting viral DNA even in the state of occult infection, thereby increasing screening efficiency and safety of the blood supply.
In Saudi Arabia, studies employing HBsAg tests for screening have found different prevalence rates of HBV infection among blood donors from various regions, including central, northwestern, and eastern provinces: 2.7–9.8% [7891011]. However, no study has examined the prevalence of HBV among blood donors in the Eastern Province of Saudi Arabia using NATs. Accordingly, the present study analyzed the prevalence of and yearly trends in HBV seromarkers among volunteer and replacement blood donors of Saudi ethnicity in this province, screening for HBsAg, anti-HBc, and HBV DNA.
In this retrospective study, data from January 2011 through December 2015 for 22,842 blood donors (N=4,494 in 2011, N=4,396 in 2012, N=4,792 in 2013, N=4,432 in 2014, and N=4,728 in 2015) were retrieved from blood bank records of King Fahd Hospital of the University of Dammam, Dammam, Saudi Arabia, which is a regional tertiary-care multi-speciality hospital. Only donors of Saudi ethnicity residing in the Eastern Province, of all age groups and both genders, were included. Expatriates and donors from other regions of Saudi Arabia were excluded. The present study was conducted as a part of routine screening as required by the blood bank associated with the hospital. As retrospective data from blood bank records were used, Institutional Review Board approval and informed consent from donors were not obtained.
HBsAg was tested using the HBsAg Qualitative II kit (Abbott, Ireland Diagnostics Division, Sligo, Ireland), which identifies the presence of IgM and IgG based on a chemiluminescent microparticle immunoassay (CMIA) run on the ARCHITECT i1000SR Immunoassay system platform (Abbott Diagnostics, Abbott Park, IL, USA), following the manufacturer's instructions. The chemiluminescent reaction was measured in relative light units (RLU) by system optics and factored in relation to cut-off (CO) values of luminescence (determined from an active calibration) to determine positivity. The ratio between the sample RLU to that of the CO was calculated (S/CO). S/CO<1.00 was considered non-reactive (NR), while S/CO≥1.00 was considered reactive (R). “NR” samples were recorded as HbsAg(−), while “R” samples were further confirmed once again using the same system. Samples were confirmed to be positive when either or both reactions were deemed to be R.
For the qualitative detection of anti-HBc, the ARCHITECT Anti-HBc II assay kit (Abbott GmbH & Co. KG, Wiesbaden, Germany) was used and run on the same system described above, according to the manufacturer's instructions. This kit identifies IgM and IgG simultaneously. The results were interpreted in the same way as described above for HBsAg. “R” samples were run an additional two times before final confirmation. A sample in which any two out of three runs showed S/CO<1.00 was flagged as “NR” and recorded as negative. A sample with S/CO≥1.00 was flagged as “R” and recorded as positive.
The NAT was performed using the Procleix Ultrio Elite assay kit (Grifols Diagnostic Solutions Inc., Los Angeles, CA, USA), as per the manufacturer's instructions. This kit is an in vitro qualitative, highly specific, robust, and sensitive system that detects the RNA of HIV-1 and hepatitis C virus (HCV) and HBV DNA. Using this kit, we performed an individual donor-NAT based on the principles of target nucleic acid capture and transcription-mediated amplification followed by the detection of amplicons by a hybridization protection assay (HPA). The chemiluminescence signals obtained during the hybridization of labeled probes on the specific amplicons were detected on a fully automated luminometer (Procleix Panther System, Grifols Diagnostic Solutions Inc.). The results were expressed as S/CO as described above. Dual kinetic assay methods [12] were used to differentiate between the signals of the internal control (provided by the kit manufacturer) and combined HIV-1/HCV/HBV signal. This kit does not discriminate between HIV-1, HCV, and HBV positivity. Thus, a result of “R” indicated that the blood sample was positive for any one of the three viruses, and the sample was further run on the HBV discriminatory assay (P/N 301109; Grifols Diagnostic Solutions Inc.) using the same kit (reagents specific for HIV-1 and HCV were excluded during the HPA stage), following the manufacturer's instructions, to determine HBV positivity.
Overall prevalence of HBV seromarkers was expressed as the percentage of seropositive samples. Categorical variables were compared using the χ2 test, and continuous variables were compared using Student's t-test. SPSS version 21.0 for Windows (IBM Corp., New York, NY, USA) was used for analyses. P<0.05 was considered statistically significant.
Yearly and cumulative data are presented in Table 1. Overall, 739 (3.24%) donors were HBV(+) as they had positive results for at least one of the markers. Of these, 676 were HBsAg(−), and 664 were only anti-HBc(+), indicating chronic or resolving infection. Further, 63 (0.28%) were positive for all three markers (Table 1). Twelve (0.05%) donors were confirmed as HBV(+) through both the anti-HBc test and NAT despite being HBsAg(−), indicating occult infection (Table 1). Prevalence as assessed by a positive result for any marker (HBsAg, anti-HBc, or HBV DNA) increased drastically (P<0.0001) from 2011 to 2012, increased marginally till 2013, and showed a decreasing trend from 2013 (P>0.05) (Fig. 1).
Screening of blood donors for HBsAg can reduce the risk of transfusion-transmitted HBV infection [13]. Post-transfusion hepatitis B occurrence has been reported earlier [14]. Isolation of anti-HBc(+) blood units has significantly contributed to reducing opportunities for post-transfusion HBV infection [15].
Our findings on the prevalence of HBsAg are similar to those reported for other regions of Saudi Arabia: 3.0% in the Saudi region of Tabuk [16], 3.8% in Jazan [17], and 3.02% in Hail [18]. However, it was higher than that of neighboring Arab countries such as Lebanon (0.9%), Egypt (1.18%), and Oman (2.8%) [13192021]. Behzad-Behbahani et al. [22] found that 6.55% of healthy donors in Iran were anti-HBc(+), 12.2% of whom also were HBV DNA(+). Ten years later, Gharib et al. [23] showed that the seroprevalence rate for anti-HBc and HBsAb in Iran was 4.9% and 31.9%, respectively. Another study conducted on randomly selected donors from Egypt revealed that 3.8% were anti-HBc IgM(+), and 18.8% were HBsAb(+) [24]. We used an HBsAg kit that can indicate the presence of both IgM and IgG. The active infection rate as confirmed by the NAT was only 0.33%, indicating overall better general health in the population as far as HBV is concerned. Nevertheless, although the presence of anti-HBc among donors may not always indicate an active infection, our donors have indications of exposure to the virus. Zekri et al. [25] reported a 1.2% HBV prevalence among blood donors in Jeddah, Saudi Arabia, while Bodhiphala et al. [26] documented this rate at 3.5% for Thai blood donors.
Blood donations during the window period of infection and occult infections could compromise the safety of the blood supply. We found that 2.91% of the donors were HBsAg(−) but anti-HBc(+), showing chronic or resolving infection. The prevalence of HBV infection in our study was inflated by a high number of donors who were anti-HBc(−) rather than by the number of HBsAg(+) donors (63/22,842).
In our study, the cases of occult HBV infections (0.05% prevalence) were identified by the NAT in donors who were anti-HBc(+) but HBsAg(−). This result emphasizes the importance of NATs in conjunction with anti-HBc tests for eliminating any residual risk of transfusion-transmitted HBV infection. Though anti-HBc reactivity alone is sufficient to determine blood units with HBV infection, it only shows a previous or resolving infection. However, NATs can identify an early infection, much before HBsAg and anti-HBc reach circulation. The present data highlights the importance of NATs for detecting early infections with non-reactive HBsAg.
The frequency of HBV positivity among blood donors, determined using a NAT, varies geographically: 0% in Turkey and Greece [272829], 1.59% in Germany [30], 7.5% in India [31], and 17.2% in Egypt [3233]. This variation could be related to the endemic nature of HBV, the sensitivity of screening tests, sample size, and type of study population.
HBV prevalence did not change substantially across five years in our study, except in the initial period between 2011 and 2012, where an increase (1.34–3.53%; P<0.0001) was observed, the reasons for which could not be ascertained. Since 2012, there have been no significant changes in HBV prevalence, although a marginal decrease can be seen since 2013. As in other populations, this trend is likely due to the introduction of mass vaccination programs against HBV [34].
Vaccinations of newborns, screening of pregnant women, screening of blood donors with advanced technologies such as NATs, and pre-employment screening of immigrants have drastically reduced the transfusion-transmitted infection rates in Saudi Arabia [34]. Our study provides valuable data to serve as a foundation for similar future studies.
A limitation of our study is that we sampled only blood donors. Our results thus may not reveal the overall prevalence of HBV in the general population of this region. Further studies should be conducted to assess the prevalence of HBV infection nationwide in the general population as well. Anti-HBc screening continues to be the key approach to identify prior HBV exposure in donors. However, only a proper donor examination with a detailed questionnaire and history followed by screening and testing will help ensure complete blood safety.
Acknowledgment
The authors greatly acknowledge the assistance received from the Blood Bank staff of King Fahad Hospital of the Imam Abdulrahman Bin Faisal University through various stages of this study. The authors are also grateful to the Deanship of Scientific Research, Imam Abdulrahman Bin Faisal University for funding this study.
References
1. World Health Organization (WHO). Fact sheets, hepatitis B Key Facts. Updated on July 2018. http://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
2. Centres for Disease Control and Prevention. World Hepatitis Day report. Updated on July 2018. http://www.cdc.gov/features/worldhepatitisday/index.html.
3. Lok AS, McMahon BJ. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Chronic hepatitis B. Hepatology. 2001; 34:1225–1241. PMID: 11732013.
4. Song Y, Bian Y, Petzold M, Ung CO. Prevalence and trend of major transfusion-transmissible infections among blood donors in Western China, 2005 through 2010. Plos One. 2014; 9:e94528. PMID: 24714490.
5. Japhet MO, Adesina OA, Donbraye E, Adewumi MO. Hepatitis B core IgM antibody (anti-HBcIgM) among hepatitis B surface antigen (HBsAg) negative blood donors in Nigeria. Virol J. 2011; 8:513. PMID: 22074048.
6. Schmidt M, Nübling CM, Scheiblauer H, Chudy M, Walch LA, Seifried E, et al. Prevalence of hepatitis B seromarkers and hepatitis C antibodies in blood donors in Basra, Iraq. BMJ Open Gastroenterol. 2016; 3:e000067.
7. Shobokshi OA, Serebour FE, Skakni L. Hepatitis B surface gene mutants and their emerging role in the efficacy of HBV vaccination programs. Ann Saudi Med. 1999; 19:87–92. PMID: 17337941.
8. Bashawri LA, Fawaz NA, Ahmad MS, Qadi AA, Almawi WY. Prevalence of seromarkers of HBV and HCV among blood donors in Eastern Saudi Arabia, 1998-2001. Clin Lab Haematol. 2004; 26:225–228. PMID: 15163322.
9. El-Hazmi MM. Prevalence of HBV, HCV, HIV-1, 2 and HTLV-I/II infections among blood donors in a teaching hospital in the Central region of Saudi Arabia. Saudi Med J. 2004; 25:26–33. PMID: 14758374.
10. El Beltagy KE, Al Balawi IA, Almuneef M, Memish ZA. Prevalence of hepatitis B virus markers among blood donors in a tertiary hospital in Tabuk, Northwestern Saudi Arabia. Int J Infect Dis. 2008; 12:495–499. PMID: 18400539.
11. Xiao X, Zhai J, Zeng J, Tian C, Wu H, Yu Y. Comparative evaluation of a triplex nucleic acid test for detection of HBV DNA, HCV RNA, and HIV-1 RNA, with the Procleix Tigris System. J Virol Methods. 2013; 187:357–361. PMID: 23142222.
12. Allain JP. Occult hepatitis B virus infection: implications in transfusion. Vox Sang. 2004; 86:83–91. PMID: 15023176.
13. El-Zayadi AR, Ibrahim EH, Badran HM, Saeid A, Moneib NA, Shemis MA, et al. Anti-HBc screening in Egyptian blood donors reduces the risk of hepatitis B virus transmission. Transfus Med. 2008; 18:55–61. PMID: 18279193.
14. Weber B, Melchior W, Gehrke R, Doerr HW, Berger A, Rabenau H. Hepatitis B virus markers in anti-HBc only positive individuals. J Med Virol. 2001; 64:312–319. PMID: 11424120.
15. Kurdi M, Abughararah M, Mulike M, Yamani O, Bugdady M, Noor M. Molecular detection of hepatitis B virus (HBV) among voluntary ELISA positive blood donors in Almadinah Almunawwarah. J Taibah Univ Med Sci. 2014; 9:166–170.
16. El Beltagy KE, Al Balawi IA, Almuneef M, Memish ZA. Prevalence of hepatitis B virus markers among blood donors in a tertiary hospital in Tabuk, Northwestern Saudi Arabia. Int J Infect Dis. 2008; 12:495–499. PMID: 18400539.
17. Mohammed Abdullah S. Prevalence of hepatitis B and C in donated blood from the Jazan region of Saudi Arabia. Malays J Med Sci. 2013; 20:41–46. PMID: 23983576.
18. Mahaba HM, El-Tayeb Ael K, El-Sekibi DK, El Gofaei AF, El-Baz HS, Ismail NA. Pattern of HBsAg positivity in selected groups at King Khalid General Hospital-Hail region, Kingdom of Saudi Arabia. J Family Community Med. 1997; 4:30–36. PMID: 23008563.
19. Mahoney FJ, Woodruff BA, Erben JJ, Coleman PJ, Reid EC, Schatz GC, et al. Effect of a hepatitis B vaccination program on the prevalence of hepatitis B virus infection. J Infect Dis. 1993; 167:203–207. PMID: 8418167.
20. Ramia S, Ramlawi F, Kanaan M, Klayme S, Naman R. Frequency and significance of antibodies against hepatitis B core (anti-HBc) antigen as the only serological marker for hepatitis B infection in Lebanese blood donors. Epidemiol Infect. 2005; 133:695–699. PMID: 16050516.
21. Kaminski G, Alnaqdy A, Al-Belushi I, Nograles J, Al-Dhahry SH. Evidence of occult hepatitis B virus infection among Omani blood donors: a preliminary study. Med Princ Pract. 2006; 15:368–372. PMID: 16888395.
22. Behzad-Behbahani A, Mafi-Nejad A, Tabei SZ, Lankarani KB, Torab A, Moaddeb A. Anti-HBc and HBV-DNA detection in blood donors negative for hepatitis B virus surface antigen in reducing risk of transfusion associated HBV infection. Indian J Med Res. 2006; 123:37–42. PMID: 16567866.
23. Karimi G, Zadsar M, Vafaei N, Sharifi Z, FalahTafti M. Prevalence of antibody to hepatitis B core antigen and hepatitis B virus DNA in HBsAg negative healthy blood donors. Virol J. 2016; 13:36. PMID: 26944046.
24. Badrawy H, Bakry R. Anti-HBc and HBV-DNA detection in blood donors negative for hepatitis B virus surface antigen. Am J Mol Biol. 2013; 3:62–66.
25. Zekri AR, Awlia AA, El Mahalawi H, Ismail EF, Mabrouk GM. Evaluation of blood units with isolated anti HBC for the presence of HBV DNA. Dis Markers. 2002; 18:107–110. PMID: 12515905.
26. Bodhiphala P, Chaturachumroenchai S, Chiewsilp P, Pruksananonda P. Detection of HBV genome by gene amplification method in HBsAg negative blood donors. J Med Assoc Thai. 1999; 82:491–495. PMID: 10443099.
27. Findik D, Arslan U, Baykan M. Determination of hepatitis B virus DNA incidence, viral load, and mutations in blood donors with HBsAg and anti-HBs-negative serology and antibodies to hepatitis B core antigen. Eur J Intern Med. 2007; 18:571–575. PMID: 18054706.
28. Arabaci F, Oldacay M. Investigation of mutant hepatitis B virus in core antibody seropositive cases of blood donor population. J Med Sci. 2008; 8:316–320.
29. Zervou EK, Dalekos GN, Boumba DS, Tsianos EV. Value of anti-HBc screening of blood donors for prevention of HBV infection: results of a 3-year prospective study in Northwestern Greece. Transfusion. 2001; 41:652–658. PMID: 11346702.
30. Schmidt M, Nübling CM, Scheiblauer H, Chudy M, Walch LA, Seifried E, et al. Anti-HBc screening of blood donors: a comparison of nine anti-HBc tests. Vox Sang. 2006; 91:237–243. PMID: 16958836.
31. Asim M, Ali R, Khan LA, Husain SA, Singla R, Kar P. Significance of anti-HBc screening of blood donors and its association with occult hepatitis B virus infection: implications for blood transfusion. Indian J Med Res. 2010; 132:312–317. PMID: 20847378.
32. Said ZN, Sayed MH, Salama II, Aboel-Magd EK, Mahmoud MH, Setouhy ME, et al. Occult hepatitis B virus infection among Egyptian blood donors. World J Hepatol. 2013; 5:64–73. PMID: 23646231.
33. Mahmoud OA, Ghazal AA, Del Metwally S, Elnour AM, Yousif GE. Detection of occult hepatitis B virus infection among blood donors in Sudan. J Egypt Public Health Assoc. 2013; 88:14–18. PMID: 23528527.
34. Aljarbou AN. The emergent concern of hepatitis B globally with special attention to Kingdom of Saudi Arabia. Int J Health Sci (Qassim). 2013; 7:333–340. PMID: 24533027.
Fig. 1
Yearly prevalence of HBV among blood donors from the Eastern Province of Saudi Arabia. Prevalence increased from 2011 through 2012 drastically (P<0.0001; χ2 test), increased marginally thereafter, and showed a decreasing trend from 2013 (P>0.05). Similar trends were noted for anti-HBc levels among donors.
*P<0.0001.
Abbreviations: HBV, hepatitis B virus; HBsAg, hepatitis B virus surface antigen; anti-HBc, antibody against hepatitis B virus core antigen; NAT, nucleic acid test.
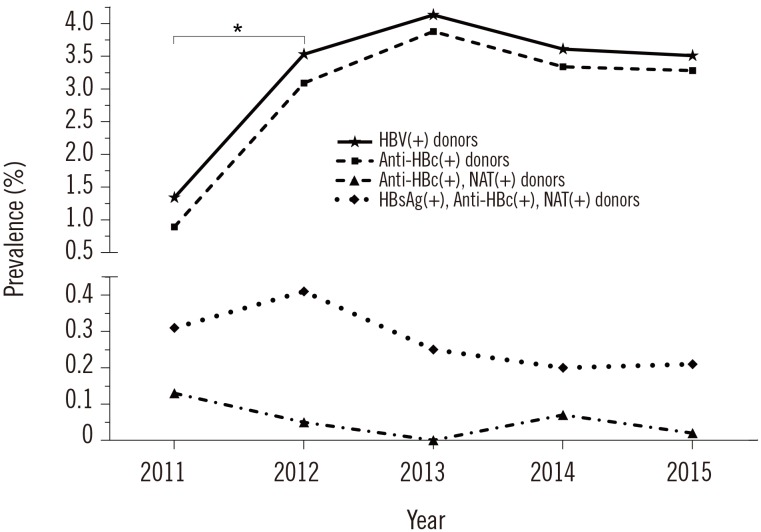
Table 1
Yearly prevalence of HBV seromarkers and HBV DNA in the Eastern Province of Saudi Arabia




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download