Abstract
Background
The Automated Fluorescent Immunoassay System (AFIAS) rotavirus assay (Boditech Med Inc., Chuncheon, Korea) is a new rapid antigen test for rotavirus detection. We evaluated the performance of this assay for detecting rotaviruses and their specific genotypes in clinical stool samples.
Methods
AFIAS rotavirus assay was performed in 103 rotavirus-positive and 103 rotavirus-negative stool samples (confirmed by both PCR and ELISA), and its results were compared with those of PCR, ELISA, and immunochromatographic assay (ICA). We evaluated diagnostic sensitivity/specificity, the detectability of rotavirus subtypes, lower limit of detection (LLOD), reproducibility, cross-reactivity, and interference of AFIAS rotavirus assay.
Results
Based on PCR and ELISA results, diagnostic sensitivity and specificity of the AFIAS rotavirus assay were both 99.0%. LLOD results showed that the AFIAS assay had sensitivity similar to or greater than ICA and ELISA. High reproducibility was confirmed, and no cross-reactivity or interference was detected. This assay could detect genotypes G1P[8], G2P[4], G3P[8], G4P[6], G4P[8], G8P[4], G8P[8], G9P[4], and G9P[8].
Conclusions
The AFIAS rotavirus assay showed high reproducibility, sensitivity, and specificity as well as excellent agreement with ELISA, PCR, and ICA. It detected the most common as well as unusual genotypes of rotavirus prevalent in Korea. It could be a useful on-site assay for rapid, convenient, and cost-effective detection of rotavirus infection.
Rotaviruses are the major cause of severe diarrheal disease in children below five years worldwide [12]. An estimated 215,000 children died from severe rotavirus-related gastroenteritis in 2013, with most of these deaths occurring in developing countries [3].
Rotaviruses has a genome of 11 double-stranded RNA segments surrounded by a triple-layered capsid consisting of a core, inner capsid, and an outer capsid layer [12]. Based on the antigenic variants of the VP6 inner capsid protein, rotaviruses can be classified into nine serogroups (A–I). Group A rotaviruses are the principal agents of human infections. The outer capsid protein consists of two structural proteins, VP7 and VP4, which are used to classify rotaviruses into the VP7 (G) and VP4 (P) genotypes. Over 27 G and 37 P genotypes have been reported to date, and the genotypic distribution of rotavirus strains shows temporal and geographic fluctuations [12].
Accurate and rapid diagnosis of rotavirus infection is important to determine appropriate treatment and to prevent the unnecessary use of antibiotics and spread of the infection. Practically, rotavirus infection can be diagnosed from stool samples by immunoassays and molecular diagnostic techniques, such as PCR. Immunoassays (immunochromatography, enzyme immunoassay, etc.) are the most widely used diagnostic methods in routine laboratories because of the rapid turn-around time and cost-effectiveness. The antibodies used in these assays are the most important factors determining detection efficiency for stool samples [45]. Monoclonal antibodies targeting the VP6 inner capsid protein, which is known to have epitopes in common with most rotavirus genotypes, are widely used in rotavirus detection immunoassays [6]. As over 27 G and 37 P genotypes of rotavirus have been reported, it is necessary to confirm that a given rotavirus detection immunoassay will be able to detect most of the numerous rotavirus genotypes. However, there is little information available on genotype detectability of commercial rotavirus immunoassays because genotype analysis of rotavirus has been performed at only a few rotavirus research laboratories, not at general clinical laboratories. Therefore, genotype detectability of rotavirus immunoassays should be evaluated.
The Automated Fluorescent Immunoassay System (AFIAS) rotavirus assay (Boditech Med Inc., Chuncheon, Korea) is a newly developed automated fluorescent lateral-flow immunoassay for the rapid detection of rotaviruses in human stool samples [7]. We evaluated its performance for detecting rotaviruses in stool samples from Korean patients and compared the results with those of other conventional assays. In addition, we examined which genotypes can be detected with the AFIAS rotavirus assay.
This study was approved by the Institutional Review Board of Hallym University, Dongtan Sacred Heart Hospital (IRB No. 2016-105). A total of 103 rotavirus-positive and 103 rotavirus-negative stored stool samples were used. The stool samples were collected from 206 gastroenteritis patients admitted to Hallym University Dontan Secred Heart Hospitals between October 2015 and March 2016. Age ranged from 0 to 90 years (median, three years); 87.4% of the positive samples were obtained from patients younger than five years. Each stool sample was diluted to a 10% stool suspension in phosphate-buffered saline and stored at −70℃ until use. The positive/negative status of these samples was confirmed by PCR (Seeplex Diarrhea-V ACE; Seegene, Seoul, Korea) and ELISA (RIDASCREEN Rotavirus test; R-Biopharm, Darmstadt, Germany). Samples determined to be positive by both PCR and ELISA were regarded as “true positive” samples, and samples determined to be negative by both methods were considered “true negative” samples. Samples that had discordant results between the two assays were excluded.
The AFIAS rotavirus assay detects the rotavirus VP6 capsid antigen in stool samples, using the AFIAS-6 analyzer system (Boditech Med Inc.) and disposable AFIAS cartridge (Boditech Med Inc.). This assay uses sandwich immunoassay with detector mAbs (Mouse Anti-Rotavirus monoclonal antibody labeled with europium chelate) in a sample pad and capture mAbs (Mouse Anti-Rotavirus monoclonal antibody) immobilized on the nitrocellulose membrane in AFIAS cartridge [7]. Fifty microliters of the diluted stool sample was added to the sample pad containing a dried fluorescence-labeled detector antibody of the cartridge, and the sample was then moved onto the nitrocellulose membrane in the cartridge by capillary action. If the target rotavirus antigens were present in the sample, they would react with the fluoresence-labeled detector antibody to form an antigen-antibody complex and would be moved and then captured by capture antibodies on the nitrocellulose membrane. After a reaction time of 12 minutes, the AFIAS-6 scanner (Boditech Med Inc.) measured the fluorescence intensity, which was expressed as a relative COI (cut-off index) value and was approximately proportional to the concentration of the rotavirus antigens in the sample. The sample result was interpreted as “positive” when the COI of the AFIAS–6 rotavirus assay was ≥1.0 and “negative” when COI was <1.0. All procedures were performed according to the manufacturer's instructions.
The Seeplex Diarrhea-V ACE detection kit was used to simultaneously detect Group A rotaviruses, adenovirus types 40 and 41 (species F), noroviruses GI and GII, and astroviruses. Viral RNA was extracted from the stool suspensions using a QIAamp Viral RNA Mini kit (Qiagen, Hilden, Germany) and the QIAcube platform (Qiagen). The nucleic acids were amplified on a PTC-200 thermal cycler (Bio-Rad, Hercules, CA, USA), and the PCR products were visualized after electrophoresis on an agarose gel. All procedures were performed according to the manufacturer's instructions.
A rotavirus antigen assay was conducted using the RIDASCREEN Rotavirus test and an automated ELISA application (GEMINI, Stratec Biomedical AG, Birkenfeld, Germany). This assay also uses monoclonal antibodies against rotavirus VP6 capsid antigens. One hundred microliters of the diluted stool suspension was added to the microwell plate, and the assay was performed according to the manufacturer's instructions. The qualitative results (positive or negative) were read based on the calculated cut-off (optical density [OD] of negative control+0.15).
The SD Bioline Rotavirus test (Standard Diagnostics, Yongin, Korea) is an ICA that automatically detects rotavirus VP6 capsid antigen in stool samples [8]. Three drops of diluted stool samples were added to the sample wells of the test device, and the results (positive or negative) were read with naked eyes after 15 minutes, according to the manufacturer's instructions.
Rotavirus G (VP7) and P (VP4) genotyping was carried out by reverse transcription (RT)-PCR and sequencing, using VP7- and VP4-specific primer sets [2]. RT-PCR was carried out with a Qiagen OneStep RT-PCR Kit (Qiagen), and PCR products were sequenced on an Applied Biosystems 3,500 Dx Genetic analyzer (Applied Biosystems, Foster City, CA, USA). Genotyping was performed by GenBank nucleotide Basic Local Alignment Search Tool (BLAST) searches (http://blast.ncbi.nlm.nih.gov/).
Negative, weak-positive, and positive controls of Group A rotaviruses were used to evaluate the reproducibility of the AFIAS rotavirus assay. Repeatability was tested in 10 replicates within a run, between-day precision was tested over five days using five replicates, and between-lot precision was tested using three different lots and five replicates per lot. The mean value and CV% of repeated tests for each concentration were calculated for within-run variation, between-day variation, and between-lot variation.
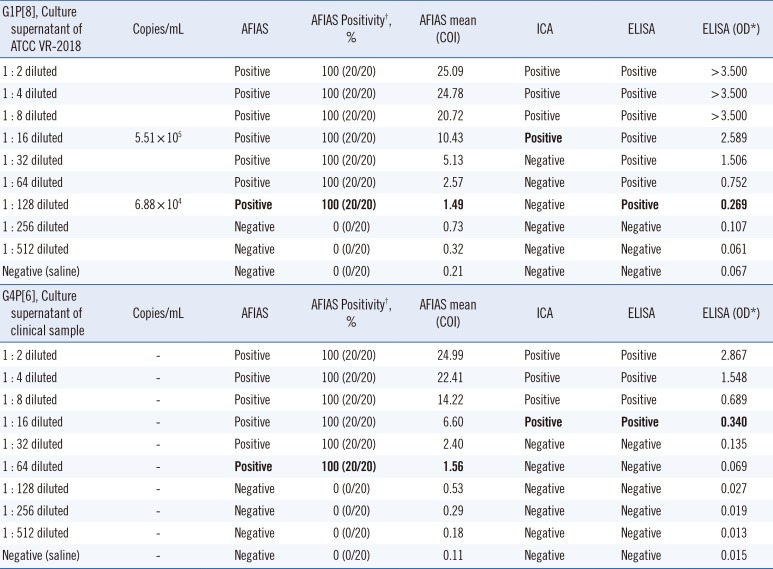
Culture supernatants of two types of rotavirus (American Type Culture Collection (ATCC) VR-2018, G1P[8] type; clinical isolate, G4P[6] type) were two-fold serially diluted with saline (1:2 to 1:512 dilutions) and used to evaluate the LLOD of the AFIAS rotavirus assay. Each diluted sample was tested in 20 replicates. The concentration of rotavirus was determined using real-time PCR (AccuPower diarrhea V1 multiplex RT-PCR kit assay, Bioneer Co., Daejeon, Korea) with in vitro-transcribed RNA calibrators, and the number of rotavirus RNA copies per milliliter was recorded for each sample. The LLOD of the AFIAS rotavirus assay was compared with that of the ICA and ELISA, using the same samples.
The potential for cross-reactivity with other viruses, bacteria, and fungi was examined using culture supernatants of the viruses and cultured colonies of the bacteria and fungi. The strains used to evaluate the cross-reactivity are shown in Table 1.
Interference tests were performed with the following substances: human blood, barium sulfate (contrast medium), hemoglobin (Sigma Aldrich Co., St. Louis, MO, USA), bilirubin (Sigma Aldrich Co.), triglyceride mix (Sigma Aldrich Co.), loperamide (anti-diarrhea drug; Janssen, Seoul, Korea), metronidazole (antibiotic; CJ Pharma, Seoul, Korea), cephradine (SCD Pharm, Seoul, Korea), cefuroxime (SCD Pharm), cefpodoxime (antibiotic; CJ Pharma), cefixime (Hanmi Pharm, Seoul, Korea), tetracycline (Chong Kun Dang Pharm, Seoul, Korea), levofloxacin (Jeil Pharm, Seoul, Korea), amoxicillin (Chong Kun Dang Pharm), ibuprofen (Samil Pharm, Yeosu, Korea), and acetaminophen (Janssen).
The degree of agreement between AFIAS and other tests was assessed. We calculated positive, negative, and total agreement rate and kappa coefficient (κ) with 95% confidence intervals (CI) (where 0.001–0.2 indicates slight agreement, 0.201–0.4 indicates fair agreement, 0.401–0.6 indicates moderate agreement, 0.601–0.8 indicates substantial concurrence, and 0.801–0.999 indicates excellent agreement). We also calculated diagnostic sensitivity and specificity. Statistical analyses were performed using Analyse-it version 2.20 (http://www.analyse-it.com/).
Table 2 shows a comparison of AFIAS rotavirus assay and ICA with PCR and ELISA. Sensitivity, specificity, and overall agreement rates of the AFIAS assay compared with PCR and ELISA were 99.0% (95% CI: 94.7–99.8%), and 99.0% (95% CI: 94.7–99.8%), and 99.0% (95% CI: 96.5–99.7%, κ=0.98), respectively. Similarly, sensitivity, specificity, and overall agreement rates of the ICA compared with PCR and ELISA were >98% (κ=0.98). The overall agreement among the four methods was 98.06%. Discrepant results among assays are shown in Table 3. Compared with ICA, AFIAS could detect an additional positive sample (G2P[4] type); however, AFIAS yielded one false-positive result (for which PCR, ELISA, and ICA yielded negative results). Both AFIAS and ICA yielded a false-negative result for one sample of the G1P[8] genotype.
The overall detectability rates of the rotavirus genotypes by AFIAS are presented in Table 4. The following genotypes were detected: G1P[8], G2P[4], G3P[8], G4P[6], G4P[8], G8P[4], G8P[8], G9P[4], G9P[8], GxP[4], and GxP[8]. One sample of G1P[8] was not detected by the AFIAS rotavirus assay; this sample had a low OD (0.507) in ELISA and was also not detected by ICA.
The repeatability and reproducibility of AFIAS are shown in Table 5. All measurements for rotavirus-negative samples showed negative results. The within-run CVs for rotavirus weak-positive and positive samples were both 3.6%. The between-day CVs for weak-positive and positive samples were 3.6 and 3.9%, and between-lot CVs were 3.9 and 4.7%, respectively.
To determine the LLOD, 2-fold serial dilutions of the rotavirus G1P[8] (8.81×106 copies/mL) and G4P[6] culture supernatants were used. For the G1P[8] genotype, all 20 replicates of the 1:128-diluted sample (6.88×104 copies/mL) were positive, while all 20 replicates of the 1:256-diluted samples tested negative by AFIAS (Table 6). Therefore, the LLOD of AFIAS was 6.88×104 copies/mL for the G1P[8] type. The concentration of the G4P[6] culture supernatant was not measured. Comparison of the LLOD among AFIAS, ICA, and ELISA showed that AFIAS was eight times more sensitive than the ICA, and its sensitivity was similar to that of ELISA for detecting the G1P[8] genotype. Moreover, the sensitivity of AFIAS was four times that of ICA and ELISA for detecting the G4P[6] genotype.
Negative signals were obtained for all 16 viruses, 28 bacteria, two fungi, and other chemicals assayed, demonstrating no cross-reactivity or interference in AFIAS (Table 1).
In our assessment of the performance of AFIAS, we found high agreement rates between AFIAS and both PCR and ELISA. As there can be discrepant results between PCR and ELISA [91011], only samples that tested positive or negative by both methods were selected for further analysis with AFIAS. The agreement rate (99.0%) between AFIAS and both PCR and ELISA was similar to or much higher than that reported in other studies [81213]. Only three samples (1.5%) showed discrepant results among the three methods: one sample (which showed a very weak-positive result in ELISA) had a false-negative AFIAS result, one AFIAS-positive sample was a false-positive as it tested negative in the other assays, and one sample with a high COI of 24.03 containing genotype G2P[4] was detected by AFIAS, but not by ICA. Potential causes of these inconsistencies include differences in the antibodies used, and hook or matrix effects of the tests [91011].
The LLOD of AFIAS was 6.88×104 copies/mL, similar to that estimated previously [9]. Interestingly, the LLOD of AFIAS was similar to that of ELISA for detecting the G1P[8] genotype, whereas AFIAS was more sensitive than ELISA for detecting the G4P[6] genotype. ICA showed the lowest sensitivity for detecting the G1P[8] genotype among the three immunoassays but showed a similar sensitivity to ELISA for detecting G4P[6]. This shows that the analytical sensitivity (LLOD) of rotavirus kits can differ among assays for detecting specific rotavirus genotypes, possibly because of differences in antibodies used [91011].
The rotavirus genotype detectability of immunoassays has been rarely studied to date. We demonstrated the diagnostic stability of the AFIAS rotavirus assay irrespective of the various rotavirus genotypes present, suggesting its applicability for the emergence or outbreak of unusual or infrequent genotypes. AFIAS detected the genotypes G1P[8], G2P[4], G3P[8], G4P[6], G4P[8], G8P [4], G8P[8], G9P[4], and G9P[8]. Although genotypes G1P[8], G2P[4], G3P[8], G4P[6], and G9P[8] are commonly detected in Korea[1], G8P[4] has not been described in previous reports or rotavirus isolates from Korea. Rotavirus infection cases with genotype G8P[4], the strain typically found in Africa and sporadically in Europe, Brazil, and Indonesia, were first reported in 2012 in the USA; this suggests the emergence of genotypes not represented by current vaccines [1415].
Although the AFIAS rotavirus assay was designed as a qualitative assay, it could roughly estimate the rotavirus titer in stool samples based on the COI (similar to the OD for ELISA). Repeatability and reproducibility of this quantitative assay were very good; its CV ranged from 3.6% to 4.7% except for the negative control, and this range is within the level acceptable for a quantitative assay (<15%) according to the US Food & Drug Administration [16]. Another advantage of the AFIAS rotavirus assay is the rapid turn-around time (12 minutes) with random access, which can be helpful in a clinical setting. This is much faster than ELISA, which usually requires two to three hrs to obtain results. In addition, AFIAS requires a smaller sample volume (50 µL) than ICA or ELISA does (100 µL). The recently developed multiplex molecular testing method is widely used because it allows for the detection of multiple pathogens simultaneously; however, the cost of such assays is very high, and they require special equipment with a longer turn-around time compared with the rapid rotavirus antigen assay. Therefore, rapid rotavirus antigen tests with high sensitivity, such as AFIAS, will remain useful tools for on-site testing, providing benefits of random access and rapid turn-around time.
In conclusion, AFIAS showed high reproducibility, sensitivity, and specificity; no interference; no cross-reactivity; and excellent agreement with ELISA, PCR, and ICA. Therefore, AFIAS can be useful in clinical practice as an on-site assay for the rapid, convenient, and cost-effective detection of rotavirus infection. Our study is also the first to report G8P[4] in Korea, which is prevalent in Africa. This finding warns researchers and clinicians of the emergence of new genotypes, which should be closely monitored with assays that detect the broad genetic diversity among global rotavirus strains.
Acknowledgements
This research was supported by the Ministry of Trade, Industry & Energy (Technology Innovation Program 10047748) and by the National Research Foundation of Korea (NRF-2017R1D1A3 B03031940). We are grateful to Bioneer Corporation for measuring the concentration of rotavirus in the culture supernatants.
References
1. Desselberger U. Rotaviruses. Virus Res. 2014; 190:75–96. PMID: 25016036.
2. Kim JS, Kim HS, Hyun J, Kim HS, Song W, Lee KM, et al. Analysis of rotavirus genotypes in Korea during 2013: an increase in the G2P[4] genotype after the introduction of rotavirus vaccines. Vaccine. 2014; 32:6396–6402. PMID: 25312273.
3. Tate JE, Burton AH, Boschi-Pinto C, Parashar UD, World Health. Global, regional, and national estimates of rotavirus mortality in children < 5 years of age, 2000-2013. Clin Infect Dis. 2016; 62:S96–S105. PMID: 27059362.
4. Miño S, Kern A, Barrandeguy M, Parreño V. Comparison of two commercial kits and an in-house ELISA for the detection of equine rotavirus in foal feces. J Virol Methods. 2015; 222:1–10. PMID: 25979610.
5. Fleming FE, Graham KL, Taniguchi K, Takada Y, Coulson BS. Rotavirus-neutralizing antibodies inhibit virus binding to integrins alpha 2 beta 1 and alpha 4 beta 1. Arch Virol. 2007; 152:1087–1101. PMID: 17318737.
6. Jalilvand S, Marashi SM, Shoja Z. Rotavirus VP6 preparations as a non-replicating vaccine candidates. Vaccine. 2015; 33:3281–3287. PMID: 26021725.
7. Ryu JH, Kwon M, Moon JD, Hwang MW, Lee JM, Park KH, et al. Development of a rapid automated fluorescent lateral flow immunoassay to detect hepatitis B surface antigen (HBsAg), antibody to HBsAg, and antibody to hepatitis C. Ann Lab Med. 2018; 38:578–584. PMID: 30027702.
8. Kim J, Kim HS, Kim HS, Kim JS, Song W, Lee KM, et al. Evaluation of an immunochromatographic assay for the rapid and simultaneous detection of rotavirus and adenovirus in stool samples. Ann Lab Med. 2014; 34:216–222. PMID: 24790909.
9. Kim HS, Kim JS. Discrepancies between antigen and polymerase chain reaction tests for the detection of rotavirus and norovirus. Ann Clin Lab Sci. 2016; 46:282–285. PMID: 27312553.
10. Tate JE, Mijatovic-Rustempasic S, Tam KI, Lyde FC, Payne DC, Szilagyi P, et al. Comparison of 2 assays for diagnosing rotavirus and evaluating vaccine effectiveness in children with gastroenteritis. Emerg Infect Dis. 2013; 19:1245–1252. PMID: 23876518.
11. Ye S, Roczo-Farkas S, Whiley D, Lambert S, Robson J, Heney C, et al. Evidence of false-positive results in a commercially available rotavirus assay in the vaccine era, Australia, 2011-2012. Euro Surveill. 2013; 18:20483. PMID: 23725979.
12. Gautam R, Lyde F, Esona MD, Quaye O, Bowen MD. Comparison of Premier™ Rotaclone®, ProSpecT™, and RIDASCREEN® rotavirus enzyme immunoassay kits for detection of rotavirus antigen in stool specimens. J Clin Virol. 2013; 58:292–294. PMID: 23850415.
13. Ye S, Lambert SB, Grimwood K, Roczo-Farkas S, Nimmo GR, Sloots TP, et al. Comparison of test specificities of commercial antigen-based assays and in-house PCR methods for detection of rotavirus in stool specimens. J Clin Microbiol. 2015; 53:295–297. PMID: 25339400.
14. Weinberg GA, Payne DC, Teel EN, Mijatovic-Rustempasic S, Bowen MD, Wikswo M, et al. First reports of human rotavirus G8P[4] gastroenteritis in the United States. J Clin Microbiol. 2012; 50:1118–1121. PMID: 22170918.
15. Ianiro G, Delogu R, Bonomo P, Castiglia P, Ruggeri FM, Fiore L. Molecular characterization of human G8P[4] rotavirus strains in Italy: proposal of a more complete subclassification of the G8 genotype in three major lineages. Infect Genet Evol. 2014; 21:129–133. PMID: 24252348.
16. US Food and Drug Administration. Bioanalytical method validation: guidance for industry. Updated on May 2018. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm070107.pdf.
Table 1
Viruses, bacteria, and fungi tested for cross-reactivity
Table 2
Comparison of AFIAS and ICA with PCR and ELISA for the detection of rotavirus in clinical stool samples
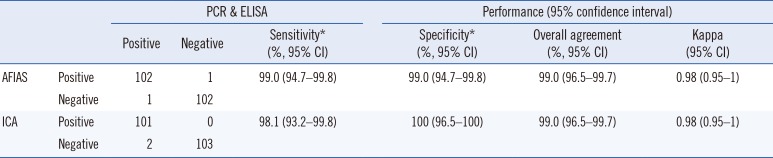
*Sensitivity and specificity were calculated considering that samples positive by both PCR and ELISA were “true positive,” and samples negative by both methods were “true negative.”
Abbreviations: CI, confidence interval; AFIAS, Automated Fluorescent Immunoassay System; ICA, immunochromatographic assay.
Table 3
Discrepant results among AFIAS, ICA, PCR, and ELISA for the detection of rotavirus in clinical stool samples
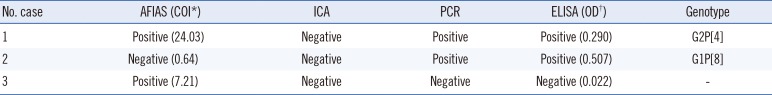
Table 4
Genotype detectability of AFIAS and ICA
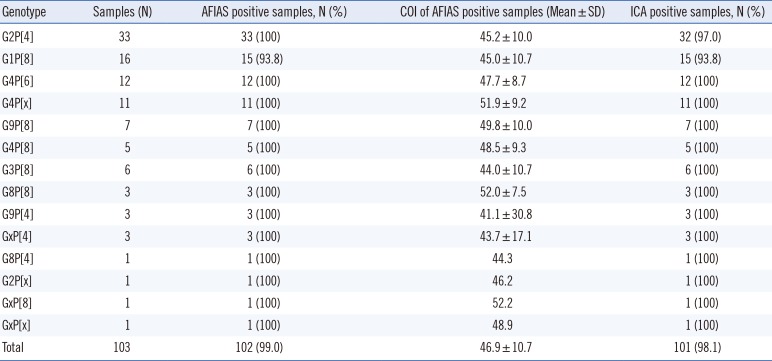
Table 5
Repeatability and reproducibility of the AFIAS rotavirus assay
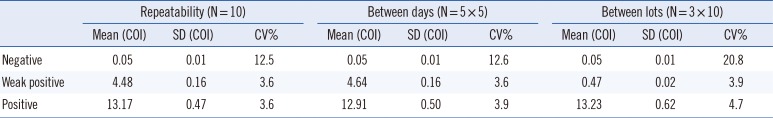
Table 6
Lower limit of detection of AFIAS for detecting G1P[8] and G4P[6] types




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download