This article has been
cited by other articles in ScienceCentral.
Abstract
A uterocutaneous fistula is rarely reported clinical condition after uterine procedures. Many diagnostic and management strategies are being suggested. In this case report, uterocutaneous fistula after pelviscopic myomectomy was diagnosed simply with hystero-salpingo contrast sonography and managed by surgical tract excision without hysterectomy and uterine wall dehiscence repair combined with medical treatment using gonadotropin-releasing hormone agonist succeeded to preserve fertility in young woman.
Keywords: Cutaneous fistula, Fertility preservation, Treatment
Introduction
Naturally, uterine fistulas such as utero-vesical or utero-colonic fisula are often seen in the patients with cervical cancer. However, uterocutaneous fistula which is a pathologic communication between adherent uterus and skin surface is a rarely reported condition. The etiologies of uterocutaneous fistula are mostly iatrogenic, such as septic abortion probably after uterine perforation [
1], previous uterine surgery including cesarean section [
2], incomplete closure of incision site and incomplete placental removal especially with abnormally invasive placental implantation [
3]. Owing to paucity of cases, there is no standard diagnostic and management strategies. Many diagnostic imaging techniques such as sonography, fistulogram, pelvis magnetic resonance imaging (MRI) and abdominal-pelvic computed tomography (CT) are diversely used according to the physicians' decisions. For management plan, major exploratory surgery including hysterectomy was used previously, but minimally invasive surgery or medical treatments are being suggested recently as an option for fertility-preservation. In this case report, we made a diagnosis with hystero-salpingo contrast sonography and managed with surgical fistula tract excision and conservative medical therapy using gonadotropin-releasing hormone (GnRH) agonist.
Case report
A 30-year-old nulliparous woman was referred to general surgery department in our tertiary medical center for suspicious small bowel ileus with possible obstruction 5 days after pelviscopic myomectomy on 10 cm sized myoma in private gynecologic hospital. On 6th day of admission, explorative laparotomy was performed due to suspected mechanical obstruction on abdominal-pelvic CT and septic condition with persistent fever and aggravated abdominal pain in spite of conservative management such as NPO, hydration and use of antibiotics.
On the surgical exploration, greenish bowel contents were found in whole intra-peritoneal cavity. Three perforation sites of proximal ileum and generally edematous and fragile small intestine from distal jejunum to proximal ileum were visible. After large amount of saline irrigation, about 1 meter long resection of small intestine and end-to-end anastomosis were performed. During the pelvic exploration, a ruptured uterine fundus was found with exposed endometrium and large hematoma was visible on the myomectomy site. Dehiscenced myometrium was repaired by interrupted 2 points suture with Vicryl 2-0 (Ethicon, Cincinnati, OH, USA).
Follow-up abdominal-pelvic CT was taken on 8 days after explorative surgery due to bloody discharge from the mid-line abdominal wound with menstrual period. It revealed large hematoma around uterine fundus and accompanied peritoneal thickening. Percutaneous abdominal drainage was inserted to the hematoma, and bacterial culture from the drainage revealed Citrobacter freundii and Enterococcus faecalis infection. After proper antibiotics treatment, the patient was discharged with afebrile condition. After twice a weekly follow-up for 1 month, the bloody discharge was noticed ongoing just during menstrual periods. Biopsy was performed on no improved abdominal wound, and pathologic report suggested endometriosis with acute and chronic inflammation with abscess formation.
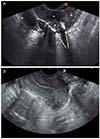
Hystero-salpingo contrast sonography was performed on the first visit in our out-patient department, 2 and a half months after initial pelviscopic myomectomy. The result revealed endometrial cavity linked to the right side of uterine fundus at which previous myomectomy was performed. Also, contrast spillage to the intra-abdominal cavity and 0.5 cm defect in the lower part of abdominal wound was observed (
Fig. 1A). Reoperation was planned for probable myometrial dehiscence repair and simultaneously uterocutaneous fistula tract removal.
Fig. 1
(A) Hysterosalpingo contrast sonography. Each marks mean follow as; Astrix - uterine outline, short arrow - EM cavity with catheter ballooning, long arrow - contrast leakage through right side of fundus to the pelvic cavity, and B - bowel. (B) postoperative sono revealing well margined myometrium.
On the explorative laparotomy performed 3 months after initial pelviscopic myomectomy, 6 cm low vertical incision was made including total removal of fistula tract opening. Fistulous tract connecting abdominal skin to the previously unhealed myomectomy site was completely resected together with surrounding soft tissues. Adhesion of small intestine, sigmoid colon, bladder dome and omentum around the fistula tract were unstucked. Percutaneous abdominal drainage site through the left lower quadrant skin puncture was also completely resected to prevent further fistula formation. Ragged uterine fundus tissue was debrided and dehiscenced uterine wall was repaired layer by layer, Vicryl 3-0 (Ethicon) interrupted suture on endometrial layer, Vicryl 2-0 (Ethicon) interrupted suture on myometrial layer.
Resected abdominal wall fistula tract specimen revealed acute and chronic inflammation with granulation tissue, extensive fibrosis, and foreign body reaction on a pathologic report. Percutaneous abdominal drainage insertion site tissue revealed chronic inflammation with extensive fibrosis, hemorrhage and foamy histocytes on a pathologic report.
Transvaginal sonography taken on 4 days after fistulectomy and uterine repair revealed well margined myometrium without hematoma and no free fluid collection in pelvic cavity (
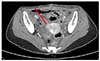
Fig. 1B). On 6 days after surgery, abdominal skin wound was well healed and stitched out. Also, follow-up abdominal-pelvic CT revealed immediate postoperative state of myomectomy site without any evidence of dehiscence (
Fig. 2). The patient was discharged with first 3.75 mg GnRH agonist (Takeda Pharmaceutical Company, Tokyo, Japan) injection. During the 3 months of GnRH agonist after the surgery, there is no evidence of recurrent fistula formation until now.
Fig. 2
Postoperative abdominal-pelvic computed tomography; immediate postoperative state without evidence of dehiscence. Arrow means sutured uterine wall.
Discussion
Although uterocutaneous fistula is a rare condition, it can be presumed with typical clinical symptoms such as bloody discharge during menstrual periods and suspicious clinical histories of uterine procedures.
Many diagnostic methods are attempted so far, and sonography is the fundamental imaging techniques. However, abdominal fistula hardly detected through transvaginal approaches. After obtaining suggestive image through transabdominal sonography, fistulogram can be used by injecting contrast-material via the skin opening [
2]. However, narrowed lumen with small cutaneous opening hole cannot be able to visualize the passage of contrast materials [
4]. Then, contrast-enhanced MRI or CT would be helpful in planning surgical intervention. More precisely, hystero-salpingo sonography with contrast injection through uterine cervix by vaginal approach can be used instead [
4]. In this case, we used hystero-salpingo sonography with contrast material via cervical opening to diagnose uterocutaneous fistula.
Since 1958 when the first case report about uterocutaneous fistula was published, a complete surgical resection including hysterectomy was the only curative method [
56] until 2007. Even medical treatment can be used to relief clinical symptoms, it did not regarded in achieving complete eradication of the fistula lesion [
7]. Therefore even bearing the risk of interval secondary surgery
, the aim of the treatment was only complete surgical excision [
8].
After 2008, medical treatment solely or combined to surgical treatment for young women who has a strongly desire fertility preserving were being reported [
39]. In these recent reports, medical treatment with GnRH agonist might atrophied fistula epithelium and spontaneous closure was achieved [
9]. In this way, uterocutaneous fistula can be conservatively treated with timely intervention without major surgery including hysterectomy. In one report, uterocutaneous fistula due to underlying peritonitis after septic abortion was completely cured with foreign body removal, irrigation, debridement and use of antibiotics. After 6 months of treatment, normal menstrual cycle was restored [
1]. Also minimally invasive laparoscopic or combined laparoscopic and laparotomic surgery after several times of repeated medical treatment reported successful clinical outcomes [
10].
In this case reports as well, including prolonged use of antibiotics until eradication of bacteremia, complete resection and debridement of fragile, necrotic, extrauterine endometriotic tissues succeed to repair uterine wall layer by layer without further dehiscence, and complete closure of abdominal wall.
Long term follow-up would be necessary to find whether use of GnRH agonist causing further atrophy of ectopic endometrial glands surely prevent recurrence of fistula or develop any sign of treatment failure. Also after the completion of medical therapy, restoration of normalized menstrual cycle and further pregnancy result should be confirmed [
11].
In conclusion, hystero-salpingo contrast sonography is inexpensive, convenient method to diagnose uterocutaneous fistula even with narrowed lumen opening. Nowadays, hysterectomy to completely eradicate fistula lesion is not the only management option any more. Proper conservative management and adjuvant medical therapy can be used as a fertility-preserving treatment option.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download