A para 2, 49-year-old woman presented with menorrhagia. Transvaginal sonography revealed 2 submucosal myomas of 3 cm each. A hysteroscopic myomectomy was planned after the administration of 3 doses of monthly gonadotrophin-releasing hormone agonists to decrease the size of the myomas. The patient's body mass index was 22.2 kg/m
2. She was taking antihypertensive drugs and denied any known drug or nondrug allergies. She had undergone an aneurysm clipping for subarachnoid hemorrhage 18 months ago and laparoscopic cholecystectomy for acute cholecystitis 16 months ago in our hospital. On admission, her vital signs were normal, including a blood pressure of 142/80 mmHg, a pulse rate of 80 beats per minute, and a body temperature of 36.9°C. Misoprostol (Cytotec
®, G.D. Searle LLC, Skokie, IL, USA) 400 μg was administered vaginally at 12:00 AM and 6:00 AM for cervical ripening prior to hysteroscopic myomectomy. Approximately 5 minutes after the second dose of vaginal misoprostol, the patient experienced uncontrolled shaking for 20 minutes; however, she did not complain of this because she thought it was just a common adverse effect of misoprostol. We routinely explain the possible adverse effects of misoprostol, including abdominal pain, vaginal bleeding, diarrhea, fever, and shivering to patients before administration of misoprostol. At 8:00 AM, the patient's body temperature was 39.0°C, and accompanying mild shivering was noted. Hydration with 300 mL of normal saline and 1 g of propacetamol hydrochloride (Denogan
®, Yungjin Pharm. Co, Ltd., Seoul, Korea) was provided intravenously to control fever. At 9:30 AM, her body temperature decreased to 37.8°C, and whole-body plethora was noted. At 9:30 AM, her blood pressure decreased abruptly to 65/40 mmHg and pulse rate increased to 125 beats per minute immediately after the induction of general endotracheal anesthesia before the start of the hysteroscopic operation. Arterial cord blood gas analysis showed a pH of 7.27 and a base deficit of −1.6 mmol/L. Oxygen saturation (SaO
2) was 98.9% (under mask O
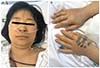
2 volume, 2 L), and the patient had a prolonged expiratory phase and a respiratory rate of 16 cycles/min, accompanied by generalized erythema, tachycardia, and hypotension. A physical examination revealed facial flushing, generalized edema, and a normal lung without evidence of oropharyngeal edema (
Fig. 1). Her hemoglobin level was 11.8 mg/dL, and there was no evidence of excessive bleeding. Hydration with 300 mL of normal saline and a volume expander was provided, and ephedrine 5 mg was administered. After 30 minutes, she became normotensive, and her blood pressure slowly increased to 120/80 mmHg. Hysteroscopic myomectomy was performed successfully without retention of the expanding medium, the normal saline. Because of concern about intracranial hemorrhage, considering her history of aneurysm rupture and clipping surgery, a tentative diagnosis of cerebral hemorrhage was made. However, brain computed tomography, performed right after the operation, showed no evidence of hemorrhage or infarction. We also ruled out cardiogenic shock on the basis of a normal electrocardiogram; normal levels of cardiac markers including creatine kinase, CK-myocardial band, B-type natriuretic peptide, and cardiac troponin-I; and a normal echocardiogram. Chest X-ray and chest computed tomography revealed no evidence of pulmonary embolism, but interstitial pulmonary edema was noted (
Fig. 2). We did not consider the possibility of an anaphylactic reaction to misoprostol and ruled out intracranial hemorrhage, pulmonary embolism, and cardiogenic shock because of the sudden unexplained hypotension. We could have administered epinephrine and diphenhydramine if we had made a tentative diagnosis of anaphylactic shock. Although we did not perform skin testing because of the patient's refusal, the patient had no allergies to any of the drugs administered before the anaphylactic shock other than the misoprostol because exactly the same drugs were used at the time of the aneurysm clipping and again at the time of cholecystectomy. A tentative diagnosis of anaphylactic shock to misoprostol was made based on case reports obtained after a PubMed search for unexplained shock performed 20 hours after the event [
5678]. The patient was in the intensive care unit for 24 hours until she was hemodynamically stable, and the generalized edema disappeared in 48 hours. On the fourth day, she was discharged without further adverse events; her condition was found to be normal without any complications at her 1- and 4-week follow-up visits.