Abstract
Background
High serum phosphate and fibroblast growth factor-23 (FGF-23) levels are well-recognized independent risk factors of mortality and morbidity in patients with chronic kidney diseases (CKDs). Sevelamer, as a phosphate chelating agent, reduces serum phosphate and FGF-23 levels produced by bone osteocytes. This study aimed to determine the best dose at which sevelamer could successfully reduce serum phosphate and FGF-23 levels in rat models of adenine-induced CKD.
Methods
CKD was induced using adenine. Healthy and CKD-induced rats were divided into 6 groups as follows: healthy controls; CKD controls; rats treated with 1%, 2%, and 3% sevelamer for CKDs; and healthy rats administered 3% sevelamer. Biochemical factors and serum FGF-23 levels were measured using spectrophotometry and enzyme-linked immunosorbent assay methods.
Results
Serum phosphate levels were best decreased in rats receiving 3% sevelamer in their diet (5.91±1.48 mg/dL vs. 8.09±1.70 mg/dL, P<0.05) compared with the CKD control rats. A dose-dependent decrease in serum FGF-23 levels was observed, and the most significant results were obtained in rats receiving 3% sevelamer compared with the CKD control rats (142.60±83.95 pg/mL vs. 297.15±131.10 pg/mL, P<0.01).
Chronic kidney disease (CKD) is a general health problem and considered as an independent risk factor for cardiovascular disease events including vascular calcifications.[12]
Fibroblast growth factor-23 (FGF-23) is a hormone produced principally by the bone osteocytes in a feedback response to increased plasma phosphate levels and acts on renal tubular cells to promote the expression of Na+-phosphate symporters and to lower the production of active vitamin D3, hence leading to increased urinary phosphate excretion and decreased intestinal phosphate absorption in normal individuals and CKD patients.[34]
Prevention of early raise in serum FGF-23 levels could possibly counteract the premature decline in serum active vitamin D3 levels and thus the impending rise in the serum parathormone levels. As evidenced by some authors, there is a positive correlation between low serum FGF-23 levels and CKD progression, vascular calcification, and mortality.[56].
Apart from nontraditional risk factors encountered in CKD patients including vitamin D deficiency and hyperparathyroidism, namely CKD-mineral and bone disorder (CKD-MBD), FGF-23 by itself has been demonstrated to correlate with vascular calcification.[78] Furthermore as displayed by recent studies, the likelihood of CKD progression, the incidence of its related complications and, overall mortality rates are all associated with the serum phosphate levels within the upper limits of normal range.[9]
It has been well recognized that high serum phosphate levels contributes to the progression of renal damage and the means to turn serum phosphate levels down have improved kidney functions.[10] Decreased nephron mass and the associated impairment in renal phosphate excretion is a cardinal feature in CKD. Phosphate retention not only leads to secondary hyperparathyroidism but also is partially responsible for the renal failure by increasing the chances of renal calcification.[11]
Growing evidence showed that rising FGF-23 levels in early stages of CKD are partially accountable for maintaining phosphatemia within the normal range.[12]
Although it is mostly assumed that high serum levels of phosphate can directly stimulate FGF-23 secretion, explicit confirming evidence is lacking. Indeed, changes in phosphate of serum did not precede changes in serum FGF-23 levels in phosphate feeding subjects.[7131415]
Several phosphate binding agents such as sevelamer hydrochloride are currently available for control of hyperphosphatemia in advanced CKD and in hemodialysis patients. The effects of sevelamer also include actions on oxidative stress, inflammation, lipid profile, endothelial dysfunction, and vascular calcification have been reported.[16]
Recent studies have shown that, sevelamer can diminish serum phosphate and consequently FGF-23 levels with high efficiency, which may contribute to its prevention of CKD progress.[1718] Additional benefactions effects of sevelamer including its ability to impede vascular calcification and alleviate bone disorders in CKD patients makes it a highly prominent therapeutic candidate.[19]
In the present study we used a rat model of adenine induced renal failure and examined the effects of different doses of sevelamer hydrochloride (1%, 2%, and 3% in diet) on serum levels of phosphate and FGF-23, and at the same time monitored the serum creatinine levels as a marker of kidney function. Our current working hypothesis is that an exponential increment in the serum levels of FGF-23 in CKD is enforced by restoring normal phosphorus homeostasis.
Ethical approval of the study was obtained from Ethical Board of Tabriz University of medical sciences, Experimental animals laboratory (IR.TBZMED.REC.1395.1084). The application form included a statement guaranteeing strict observance to the animals' rights. Attention to this rule was paid throughout the study.
Male Wistar rats weighing 226±31 g were obtained from the Laboratory Animal Center of Faculty of Medicine. Animals were kept in sterilized cages at 24±2℃ with 12-hr cycles of light and dark along with free access to water and standard pellet (normal diet, CE-2).
Renal failure was induced in male rats by feeding 0.75% adenine-containing diet for 4 weeks.[20] After an acclimatization period of 1 week, they were divided into 6 groups. The first group continued to receive the same diet without treatment until the end of the study (Normal group, n=8). The groups 2, 3, 4, and 5 were switched to a diet containing 0.75% adenine (Sigma Chemical Co., St. Louis, MO, USA). At the day 28 after the onset of diet containing adenine, tail vein blood samples were collected to measure serum levels of creatinine, and phosphate to verify the induction of CKD.
Then again induced CKD rats were divided into 4 groups (groups 2-5) that were matched for body weight, serum phosphate, calcium, and creatinine; and next were given diets with 0% sevelamer (n=10), 1% sevelamer (n=8), 2% sevelamer (n=8), 3% sevelamer (n=8) for 4 weeks. Sevelamer was mixed into normal diet. The rats in group 6 (n=10) were normal animals without receiving adenine but fed 3% sevelamer (Fig. 1). The quantities of feed eaten by each group were recorded each day. At the week 4 after onset of sevelamer, animals were weighed and anesthesia was performed using a single dose of ketamine (100 mg/kg, i.p.). Venous blood was collected and after clotting, samples were centrifuged at 3,000 g for 15 min and the separated serums were kept at −80℃ until tested.
Serum creatinine, calcium, and phosphate levels were measured using an automatic serum analyzer (Cobas Mira Plus Chemistry System; Roche Diagnostic Systems, Indianapolis, IN, USA). Calcium×phosphate product, an indicator of metastatic calcification was calculated. Serum FGF-23 levels were measured using an enzyme-linked immunosorbent assay kit (BT, Bioassay Technology laboratory).
Data were analyzed using SPSS (SPSS Inc., Chicago, IL, USA) and expressed as mean±standard deviation (SD). An unpaired t-test was used to compare the adenine-control group with the normal-control group. Differences between groups were analyzed using a one-way analysis of variance (ANOVA), and Dunnett's test. P<0.05 values were considered to be significant. Association between parameters was analyzed using the correlation coefficient analysis.
There were no significant differences in average daily food intake during the study between treatment and CKD control groups. No significant changes in animal weights were seen after switching to diets containing adenine or sevelamer. CKD rats had significantly lower weights as compared to normal ones and this difference dramatized with the progress of study (Table 1).
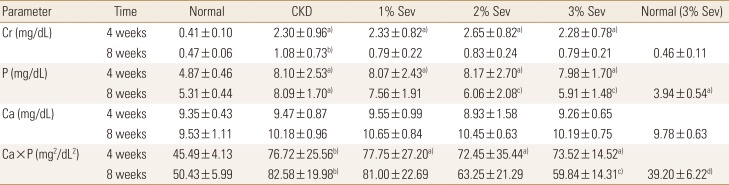
Serum creatinine and phosphate levels were higher in rats receiving adenine compared with normal controls, while serum calcium levels were nearly the same among those groups. At the end of the study, groups receiving sevelamer demonstrated still higher levels of serum creatinine, nonetheless a slight albeit statistically insignificant decrease in their levels was noted (Table 2).
As expected, CKD rats had significantly higher levels of phosphate, FGF-23, and calcium×phosphate product. sevelamer dose-dependently decreased serum levels of phosphate; though as noted in Table 2, statistically relevant reduction only was seen at doses of 2% and 3% sevelamer in diet. Serum calcium levels were nearly constant throughout the study and no significant change was observed before and after sevelamer treatment in CKD groups (Table 2). Sevelamer treatment had significant effect on calcium×phosphate product in 3% sevelamer treat.
As shown in Table 3, CKD group had high levels of FGF-23 and 1%, 2%, and 3% sevelamer treatment could successfully decrease its levels in a dose-dependent manner, worthy to say that the most significant results obtained in 3% dose of sevelamer compared to CKD group (142.60±83.95 pg/mL vs. 297.15±131.10 pg/mL, P<0.01). We found that FGF-23 is positively correlated with serum phosphate (r=0.43, P=0.01) and also were observed a significant positive correlation among FGF-23 and the Ca×P product, (r=0.36, P=0.04) (Fig. 2, 3). calcium×phosphate product is a parameter to evaluate the risk of extra skeletal calcification in CKD patients. Serum FGF-23 is not correlated with serum calcium.
The present study emphasize many previous studies that showed rising serum FGF-23 levels in early stages of CKD are partially responsible for maintaining phosphatemia. Sevelamer successfully reduced serum levels of phosphate at doses of 2% and 3% in diet. Sevelamer dose-dependently decreased serum FGF-23 levels in rat models of renal failure. Sevelamer could not significantly reduce the serum levels of creatinine after 4 weeks of administration.
In our study serum levels of creatinine were decreased with sevelamer treatment in CKD rats but this reduction was not statistically significant in agreement with the study conducted by Nagano et al.[21] in 2006. This phenomenon could possibly be attributed to the short duration of our study. Though as the treatment duration in the study conducted by Nagano et al.[22] lasted for 2 month, it could be inferred that sevelamer alone is not sufficient for significant renal functioning improvement. Nevertheless one study done in 2003 with two 1% and 3% doses of sevelamer with a treatment duration of 58 days has reported that statistically significant reduction in serum BUN and creatinine levels were achieved in induced CKD models.
The results of present study showed that, serum levels of phosphate were dose-dependently decreased with 1%, 2%, and 3% sevelamer treatment; however, statistically significant reduction was not seen in 1% sevelamer group. As opposed to our result, Nagano et al.[2122] reported that 1% sevelamer successfully reduced serum phosphate levels to the extent that prevented hyperphosphatemia in the both 2003 and 2006 studies. The extent of impairment in kidney function also was higher in our study as indicated by higher levels of creatinine compared with mentioned studies.
Treatment with 3% sevelamer led to reduce the serum phosphate levels with no changes in serum calcium levels that was accompanied by a decline in calcium×phosphate product, an indicator of metastatic calcification; the finding that emphasizes the benefits of 3% sevelamer over other to 1% and 2% doses. Significant positive correlation of FGF-23 also with the serum phosphorus and calcium×phosphate product were observed.
In accordance with the results obtained in several previously published papers,[18212223] raising in serum FGF-23 levels resulting from adenine-induced CKD, were significantly reduced after sevelamer treatment in our study in a dose dependent manner; albeit FGF-23-lowering effect of sevelamer was only noted in CKD groups, and normal animals receiving sevelamer demonstrated reduced serum phosphate levels, yet had nearly normal serum FGF-23. The explanation could be the one as follows: FGF-23 down regulates the expression of 1-α-hydroxylase in the kidney, leading to reduced levels of circulating active vitamin D in the blood, the outcome of which is reduced intestinal absorption of phosphate.[24] It seems that normal homeostasis of phosphate and FGF-23 in healthy animals is responsible for the constant levels of FGF-23.
Contradictory results has been obtained regarding the relationship between phosphate and FGF-23 levels in previous studies; for instance rats undergone 5/6 nephrectomy displayed high blood levels of FGF-23 despite receiving a phosphorus-free diet, while the expression of FGF-23 did elevated in cultured osteoblasts in response to incubation with accumulative loads of phosphorus.[25] These findings may propose the existence of phosphate independent regulation mechanism for FGF-23 production. Aside from studies mentioned, CKD patients treated with sevelamer carbonate did not show any significant variations in plasma FGF-23 levels while a significant decrease in serum phosphate levels was noted in these patients.[26]
In support of proceeding results, study conducted by Yilmaz et al.[27] illustrated that the variations seen in serum FGF-23 levels induced by phosphate chelators are accompanied by simultaneous alterations in flow-mediated vasodilatation independently of phosphate levels in serum, and therefore it is possible that an advanced hyperphosphatemia in conjunction with high levels of FGF-23, may not be mitigated by phosphate reduction alone in CKD patients.[26]
Ketteler and Biggar [28] prospective randomized trial in moderate CKD patients with the upper normal range phosphate levels showed only moderate reductions in serum phosphate levels and no effects on serum FGF-23, but increased vascular calcification progression with active treatment versus placebo.
Another even more conflicting study published in 2010 by Oliveira et al.[3] showed that sevelamer is able to decline serum FGF-23 without affecting serum phosphate levels in normo-phosphatemic pre-dialysis CKD patients. Nevertheless it is worthy to note that these results challenge the phosphate-binding nature of sevelamer and as evidenced in our study, sevelamer when administered at doses of 2% and 3% significantly reduced serum phosphate levels, though in normal rats no meaningful effect was observed on serum FGF-23 levels. Bearing in mind all the results presented earlier, it appears necessary to apprehend fully the underlying mechanisms related to serum phosphate and FGF-23 regulation in CKD patients.[29]
Yokota et al.[29] came to the conclusion by computational simulations that high FGF-23 levels in CKD patients is not a cause rather is an outcome of an ineffective FGF-23-mediated phosphorus removal and thereby rejected the assumption that the elevated level of FGF-23 in serum in CKD is a direct cause of hyperphosphatemia. In Yokota mathematical model use of FGF-23 antibody in CKD did not result in removing phosphorous burden and herein do not backing the notion that the rising serum levels of FGF-23 in CKD is a direct cause of hyperphosphotemia.
The accurate mechanism of FGF-23 regulation in CKD is not known, and further basic and clinical studies are necessary. It is needful to figure out the mechanism in CKD in which the level of FGF-23 is high without effectively regulating of phosphorus.
In conclusion, 3% sevelamer significantly reduced serum levels of phosphate, calcium×phosphate product and FGF-23 and is recommended over the 1% and 2% doses in diet. Even at this higher dose of 3% Sevelamer, no significant reduction in serum levels of creatinine, the marker of kidney function, was noted.
ACKNOWLEDGMENTS
This work is funded by a PhD grant of the Biotechnology Research Center, Tabriz University of Medical Sciences.
References
1. Denker M, Boyle S, Anderson AH, et al. Chronic renal insufficiency cohort study (CRIC): overview and summary of selected findings. Clin J Am Soc Nephrol. 2015; 10:2073–2083. PMID: 26265715.

2. Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004; 15:1307–1315. PMID: 15100371.

3. Oliveira RB, Cancela AL, Graciolli FG, et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy. Clin J Am Soc Nephrol. 2010; 5:286–291. PMID: 19965540.

4. Munoz Mendoza J, Isakova T, Ricardo AC, et al. Fibroblast growth factor 23 and Inflammation in CKD. Clin J Am Soc Nephrol. 2012; 7:1155–1162. PMID: 22554719.

5. Gutiérrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008; 359:584–592. PMID: 18687639.

6. Wesseling-Perry K. FGF23: is it ready for prime time? Clin Chem. 2011; 57:1476–1477. PMID: 21914788.

7. Burnett SM, Gunawardene SC, Bringhurst FR, et al. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006; 21:1187–1196. PMID: 16869716.

8. Muñoz-García B, Martín-Ventura JL, Martínez E, et al. Fn14 is upregulated in cytokine-stimulated vascular smooth muscle cells and is expressed in human carotid atherosclerotic plaques: modulation by atorvastatin. Stroke. 2006; 37:2044–2053. PMID: 16809572.
9. Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011; 305:2432–2439. PMID: 21673295.

10. Gutiérrez OM, Wolf M, Taylor EN. Fibroblast growth factor 23, cardiovascular disease risk factors, and phosphorus intake in the health professionals follow-up study. Clin J Am Soc Nephrol. 2011; 6:2871–2878. PMID: 22034506.

11. Cozzolino M, Dusso AS, Liapis H, et al. The effects of sevelamer hydrochloride and calcium carbonate on kidney calcification in uremic rats. J Am Soc Nephrol. 2002; 13:2299–2308. PMID: 12191974.

12. Ketteler M, Petermann AT. Phosphate and FGF23 in early CKD: on how to tackle an invisible foe. Nephrol Dial Transplant. 2011; 26:2430–2432. PMID: 21803732.

13. Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab. 2005; 90:1519–1524. PMID: 15613425.

14. Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006; 91:3144–3149. PMID: 16735491.

15. Ito N, Fukumoto S, Takeuchi Y, et al. Effect of acute changes of serum phosphate on fibroblast growth factor (FGF)23 levels in humans. J Bone Miner Metab. 2007; 25:419–422. PMID: 17968495.

16. Rodríguez-Osorio L, Zambrano DP, Gracia-Iguacel C, et al. Use of sevelamer in chronic kidney disease: beyond phosphorus control. Nefrologia. 2015; 35:207–217. PMID: 26300515.

17. Koiwa F, Kazama JJ, Tokumoto A, et al. Sevelamer hydrochloride and calcium bicarbonate reduce serum fibroblast growth factor 23 levels in dialysis patients. Ther Apher Dial. 2005; 9:336–339. PMID: 16076378.

18. Nagano N, Miyata S, Abe M, et al. Effects of intermittent treatment with sevelamer hydrochloride on parathyroid hyperplasia and vascular calcification in rats with chronic kidney disease. Clin Calcium. 2005; 15(Suppl 1):35–39. discussion 9-40.
19. Spaia S. Phosphate binders: sevelamer in the prevention and treatment of hyperphosphataemia in chronic renal failure. Hippokratia. 2011; 15:22–26.
20. Tamagaki K, Yuan Q, Ohkawa H, et al. Severe hyperparathyroidism with bone abnormalities and metastatic calcification in rats with adenine-induced uraemia. Nephrol Dial Transplant. 2006; 21:651–659. PMID: 16311258.

21. Nagano N, Miyata S, Abe M, et al. Effect of manipulating serum phosphorus with phosphate binder on circulating PTH and FGF23 in renal failure rats. Kidney Int. 2006; 69:531–537. PMID: 16395276.

22. Nagano N, Miyata S, Obana S, et al. Sevelamer hydrochloride, a phosphate binder, protects against deterioration of renal function in rats with progressive chronic renal insufficiency. Nephrol Dial Transplant. 2003; 18:2014–2023. PMID: 13679475.

23. Cancela AL, Oliveira RB, Graciolli FG, et al. Fibroblast growth factor 23 in hemodialysis patients: effects of phosphate binder, calcitriol and calcium concentration in the dialysate. Nephron Clin Pract. 2011; 117:c74–c82. PMID: 20689328.

24. Bansal S. New insights into regulation of FGF23 in chronic kidney disease and its role in cardiovascular disease. SM J Cardiolog and Cardiovasc Disord. 2015; 1:1003.
25. Liu S, Tang W, Zhou J, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006; 17:1305–1315. PMID: 16597685.

26. Spatz C, Roe K, Lehman E, et al. Effect of a non-calcium-based phosphate binder on fibroblast growth factor 23 in chronic kidney disease. Nephron Clin Pract. 2013; 123:61–66. PMID: 23774446.

27. Yilmaz MI, Sonmez A, Saglam M, et al. Comparison of calcium acetate and sevelamer on vascular function and fibroblast growth factor 23 in CKD patients: a randomized clinical trial. Am J Kidney Dis. 2012; 59:177–185. PMID: 22137672.

28. Ketteler M, Biggar PH. Use of phosphate binders in chronic kidney disease. Curr Opin Nephrol Hypertens. 2013; 22:413–420. PMID: 23736841.

Fig. 1
The experimental design. From 0 week to 4 weeks, adenine diet was administered for the four groups (CKD control group, 1%, 2%, or 3 % sevelamer administered groups). At day 28, adenine diet was stopped and after that normal diet was used. At day 28 the administration of sevelamer was started and sevelamer was administered at 1%, 2%, or 3 % mixture diet for 4 weeks. Next group included normal rats, fed 3% sevelamer. CKD, chronic kidney disease.
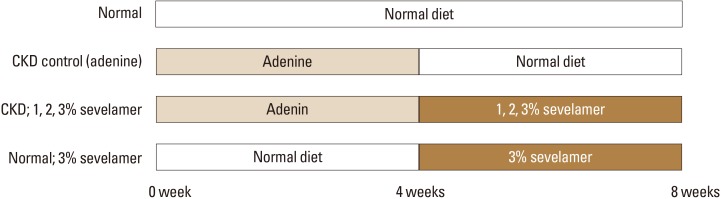
Fig. 2
Correlation between serum FGF-23 and phosphate in chronic kidney disease and sevelamer treated groups. FGF-23, fibroblast growth factor-23.
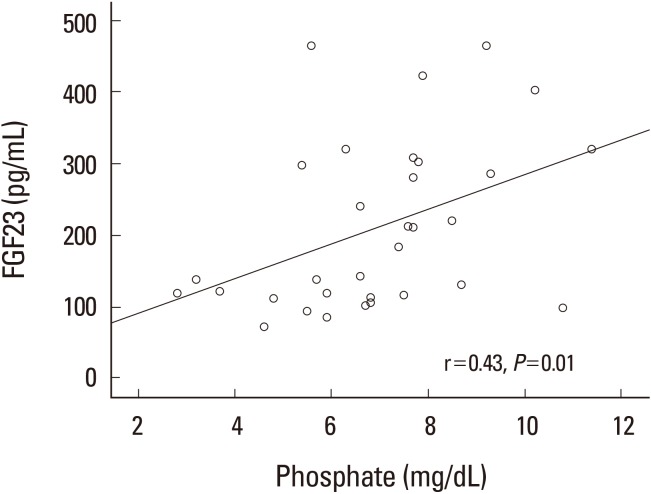
Fig. 3
Relation between FGF-23 and Ca×P product in chronic kidney disease and sevelamer treat groups. FGF-23, fibroblast growth factor-23; Ca, calcium; P, phosphorus.
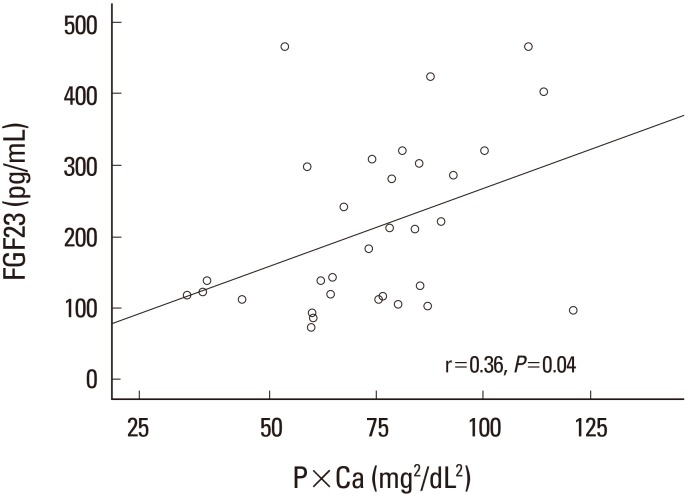
Table 2
Changes of serum biochemical parameters before and after sevelamer administration in all study groups




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download