Abstract
Purpose:
Urogenital tuberculosis (UGT) is rarely reported in developed countries. This study evaluated the genetic susceptibility of Korean patients to UGT.
Materials and Methods:
A total of 35 UGT patients who were confirmed pathologically, 44 intrapulmonary tuberculosis (IPT) patients who were confirmed radiologically, and 102 controls over a 6 year period were enrolled in this study. The region of rs2243268 in interleukin-4 (IL-4) gene was amplified from whole blood samples, and the DNA sequences were read using the Sanger method.
Results:
Twenty women and 15 men were diagnosed with UGT. The occurrence of the CC, AC, and AA genotypes of rs2243268 were 26 (74.3%), 8 (22.9%), and 1 (2.9%), respectively, in UGT; 28 (63.6%), 15 (34.1%), and 1 (2.3%), respectively, in IPT; and 51 (50.0%), 45 (44.1%), and 6 (5.9%), respectively, in the control groups (p=0.115). The bivariate data of CC and AC/AA were 74.3% and 25.7% in UGT, 63.6% and 36.4% in IPT, and 50.0% and 50.0% in the control groups, respectively (p=0.029). The UGT was significantly different from the control group among the three genotypes (p=0.038, Fisher's exact test) and bivariate genotypes (p=0.017, Fisher's exact test). In addition, people carrying the CC genotype had a higher risk of UGT (odds ratios, 2.889; 95% confidence intervals, 1.233-6.770; p=0.015).
Go to : 
REFERENCES
1.Chernyaeva E., Rotkevich M., Krasheninnikova K., Yurchenko A., Vyazovaya A., Mokrousov I, et al. Whole-genome analysis of mycobacterium tuberculosis from patients with tuberculous spondylitis, Russia. Emerg Infect Dis. 2018. 24:579–83.
2.Turner RD., Chiu C., Churchyard GJ., Esmail H., Lewinsohn DM., Gandhi NR, et al. Tuberculosis infectiousness and host susceptibility. J Infect Dis. 2017. 216(suppl_6):S636–43.

3.Harries AD. Tuberculosis and human immunodeficiency virus infection in developing countries. Lancet. 1990. 335:387–90.

4.Chandrasekaran P., Saravanan N., Bethunaickan R., Tripathy S. Malnutrition: modulator of immune responses in tuberculosis. Front Immunol. 2017. 8:1316.

5.Cantwell MF., McKenna MT., McCray E., Onorato IM. Tuberculosis and race/ethnicity in the United States: impact of socioeconomic status. Am J Respir Crit Care Med. 1998. 157:1016–20.
6.Figueiredo AA., Lucon AM. Urogenital tuberculosis: update and review of 8961 cases from the world literature. Rev Urol. 2008. 10:207–17.
7.Qi H., Sun L., Jin YQ., Shen C., Chu P., Wang SF, et al. rs2243268 and rs2243274 of interleukin-4 (IL-4) gene are associated with reduced risk for extrapulmonary and severe tuberculosis in Chinese Han children. Infect Genet Evol. 2014. 23:121–8.

8.Sivangala R., Ponnana M., Thada S., Joshi L., Ansari S., Hussain H, et al. Association of cytokine gene polymorphisms in patients with tuberculosis and their household contacts. Scand J Immunol. 2014. 79:197–205.

9.Chatterjee S., Talaat KR., van Seventer EE., Feng CG., Scott AL., Jedlicka A, et al. Mycobacteria induce TPL-2 mediated IL-10 in IL-4-generated alternatively activated macrophages. PLoS One. 2017. 12:e0179701.

10.Song J., Park H., Lee G. Contribution of genetic variation rs266882 to prostate-specific antigen levels in healthy controls with serum PSA below 2.0 ng/ml. Biochem Genet. 2013. 51:264–74.
11.Lee JY. Diagnosis and treatment of extrapulmonary tuberculosis. Tuberc Respir Dis (Seoul). 2015. 78:47–55.

12.Peto HM., Pratt RH., Harrington TA., LoBue PA., Armstrong LR. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis. 2009. 49:1350–7.

13.Pehme L., Hollo V., Rahu M., Altraja A. Tuberculosis during fundamental societal changes in Estonia with special reference to extrapulmonary manifestations. Chest. 2005. 127:1289–95.

Go to : 
 | Fig. 1.Single nucleotide polymorphism in rs2243268. Sequencing chromatogram revealed the CC genotype (left), C/A genotype (middle), and AA genotype (right). |
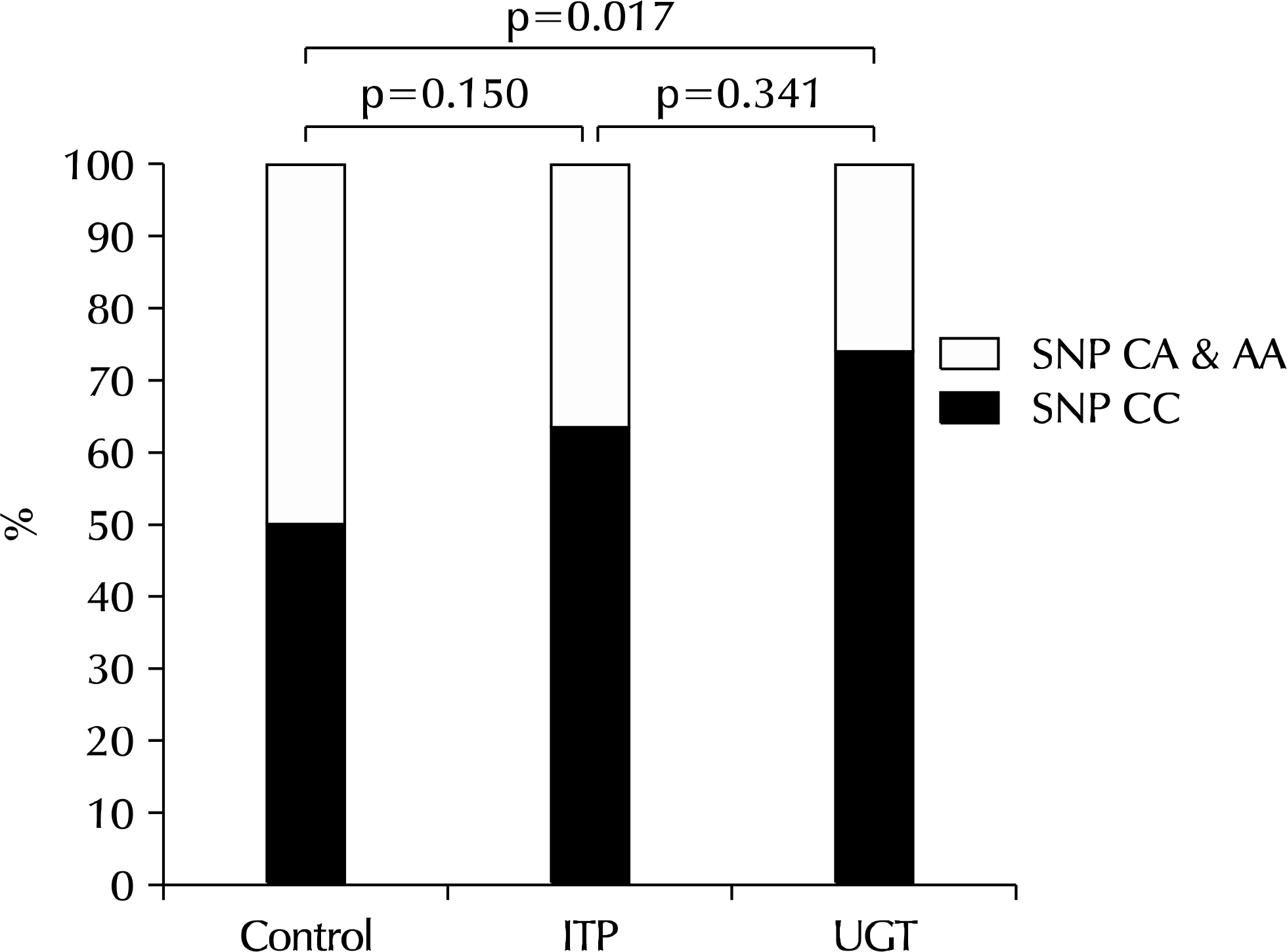 | Fig. 2.Significant differences in bivariate genotypes were observed between the urogenital tuberculosis (UGT) and the control group (p=0.017, Fisher's exact test). People carrying the CC genotype of rs2243268 had an increased risk of UGT (odds ratios, 2.889; 95% confidence intervals, 1.233-6.770; p=0.015) compared to those with the CA/AA genotypes. SNP: single-nucleotide polymorphism, IPT: intrapulmonary tuberculosis. |
Table 1.
Clinical characteristics and genotype distribution of the rs2243268 polymorphism among urogenital tuberculosis (UGT), intrapulmonary tuberculosis (IPT), and control groups




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download