Abstract
Purpose
Organophosphates, commonly used in agricultural pesticides, pose high risks and incidences of poisoning. In the present study, we investigated the relative risk and clinical severity, including laboratory results, of non-oral route poisoning (NORP) patients, compared to oral route poisoning (ORP) patients.
Materials and Methods
A single institutional toxicology database registry was utilized to gain information on clinical laboratory results on organophosphate poisoning patients who visited the emergency department (ED) between January 2000 and October 2016. Clinical outcomes, such as mortality and complication rates, were compared using 1:2 propensity score matching in the total cohort.
Results
Among a total of 273 patients in our study, 34 experienced NORP. After 1:2 propensity score matching, rates of respiratory complications and mortality were higher in the ORP group than in the NORP group. However, there was no difference in hospitalization time and time spent in the intensive care unit between the two groups. Compared with ORP patients after matching, the relative risk of mortality in NORP patients was 0.34, and the risk of respiratory distress was 0.47. The mean level of pseudocholinesterase was significantly higher in the NORP group than in the ORP group, while recovery rates were similar between the two groups.
Conclusion
Although the majority of NORP patients were admitted to the ED with unintentional poisoning and the relative risk of NORP was lower than that for ORP, we concluded that NORP is as critical as ORP. Considerable medical observation and intensive therapeutic approaches are also needed for NORP patients.
Approximately 200000 to 250000 mortalities occur annually worldwide due to poisoning by organophosphate pesticides.12 The use of organophosphates is regulated by law in many nations. Although there are regulatory laws for the use of pesticides in Korea, the toxicity of organophosphate pesticides are overlooked or incorrectly classified.3 According to the pesticide database from the Korean Rural Development Administration, many products with organophosphate components are not classified as deadly toxic agents, but as general toxic agents.3 These pesticides are strong anti-cholinergic agents, causing severe combination toxidromes, such as DUMBELS (defecation, urination, muscle weakness, bradycardia, bronchorrhea, bronchospasm, emesis, lacrimation, salivation). Thus, there is a high risk and incidence of poisoning-related mortality.
Due to high diagnostic sensitivity, serum pseudocholinesterase levels rather than serum acetylcholinesterase levels have been used as a diagnostic and prognostic marker for organophosphate poisoning.2 However, a low pseudocholinesterase level does not appear to have an accurate prognostic value in acute organophosphate poisoning.4 Organophosphate pesticide poisoning may occur by not only intentional oral ingestion, but also unintentional exposure associated with contact and inhalation. In Korea, local workers, at times, work with pesticides in forested areas for pest or insect control. The authors of this study have encountered numerous cases of poisoning via non-oral routes. While previous studies have reported adverse effects of exposure to organophosphate pesticides via the skin or inhalation, reports of fatality are rare.2 However, we have come across cases of fatal non-oral route poisoning (NORP), as well as oral route poisoning (ORP). This study was performed to investigate the relative risk and clinical severity of NORP in comparison to ORP.
This study analyzed data from organophosphate poisoning patients visiting the emergency department (ED) of Wonju Severance Christian Hospital in the Republic of Korea between January 2000 and October 2016. The emergency medical center of this tertiary hospital serves approximately 43000 ED patients annually. The inclusion criteria were patients with 1) organophosphate poisoning by self-harm history or unintentional exposure to organophosphate pesticides, 2) cholinergic symptoms and signs like DUMBELS, and 3) a serum pseudocholinesterase level lower than 3500 U/L (normal reference range: 7000–19000 U/L). Patients who did not satisfy any one of the inclusion criteria were excluded from the study. Other exclusion criteria were age younger than 18 years and co-exposure to other toxic substances, except alcohol ingestion. We confirmed information regarding the route of poisoning from the patients or their relatives. All ORP patients received gastric irrigation and charcoal administration. Atropine (Atropine inj, JEIL Pharmaceutical, Daegu, Korea) dosage was adjusted according to cholinergic symptoms. Patients also received pralidoxime (PAM-A inj, JW Pharmaceutical, Seoul, Korea) injections for 72 hours (2-PAM loading IV dose: 1 g mixed with normal saline 100 cc for 30 minutes, 2-PAM maintain IV dose: 10 g mixed with normal saline 1000 cc for 24 hours, 3 days). The indications for mechanical ventilation with endotracheal intubation were as follows: excessive bronchial secretions, poor oxygenation, decreased mentality, and severe irritability. The follow-up routine until the end of the treatment included daily blood sample tests, including serum pseudocholinesterase levels.
We conducted an observational cohort study with a retrospective analysis of patient medical records and our toxicology database registry. First, we divided the enrolled patients according to the route of organophosphate poisoning. The following data were assessed from each group: age; gender; initial vital signs (systolic blood pressure, diastolic blood pressure, pulse rate, respiratory rate, and body temperature); route of poisoning; estimated ingestion amount (if ORP); time interval from poisoning to ED arrival; initial Glasgow Coma Scale (GCS) score; and treatments, including gastric lavage, charcoal injection, atropine injection, and pralidoxime infusion. The estimated amounts of ingestion were defined as “a little” or “a spoonful” (5 cc), “a mouthful” (25 cc), “a small cup” (100 cc), and “a bottle” (300 cc).5
To compare the severity of poisoning in the enrolled patients, we assessed clinical data on rates of aspiration pneumonia, pulmonary edema, respiratory distress, hypotension (systolic blood pressure <90 mm Hg or diastolic blood pressure <60 mm Hg), central nervous system (CNS) deficits (consciousness below stupor or seizure activity), mortality, and hospitalization, as well as hospitalization time, intensive care unit (ICU) stay, and serial changes in serum pseudocholinesterase levels between the NORP and ORP groups. Acute Physiology and Chronic Health Evaluation II (APACHE II) scores were calculated using initial ED laboratory values. The overall demographics, clinical complications, and laboratory values were compared using 1:2 (NORP:ORP) propensity score matching. Our research plan was approved by the Institutional Review Board committee of Wonju College of Medicine, Yonsei University (Approval number: YWMR-13-5-031).
Statistical analysis was performed by independent sample ttests and chi-square tests. Results were expressed as mean±standard deviation. To adjust for confounding results, propensity score matching was performed. A propensity score for the predicted probability of an NORP group was estimated with the use of a multivariable logistic regression model fit. The C-statistic of the logistic regression model for propensity score matching was 0.78. The adjusted covariates in propensity score matching included organophosphate type, age, sex, systolic blood pressure, and respiratory rate. By using a nearest neighbor matching algorithm with a “greedy” heuristic, each patient in the NORP group (n=34) was matched to a maximum of two subjects in the ORP group. For this matching, SAS macro “PSMatching” was used. After propensity score matching, the groups showed no differences in organophosphate type, age, sex, systolic blood pressure, and respiratory rate. Relative risk estimation and linear mixed model were used in the analysis. Relative risk was estimated by the Genmod procedure. A linear mixed model was analyzed by SPSS (IBM Statistics for Windows, Version 23.0, IBM Corp., Armonk, NY, USA). A level of p<0.05 was considered significant. Data were analyzed on SAS version 9.3 (SAS Institute Inc., Cary, NC, USA). All statistical processing and contents were analyzed by a biostatistician.
A total of 273 patients were included in this study. Table 1 summarizes various types of agents that were implicated in the poisoning incidents; the ingredients were undetermined in 100 cases of poisoning. Among the enrolled patients, 34 were exposed to organophosphates via inhalation or dermal contact, and 239 were exposed thereto via the oral route. Thirty NORP and 28 ORP patients had been accidentally exposed. A total of 205 (75.1%) were intentionally exposed to organophosphates, and 204 of these patients were exposed by the oral route. The mean ED arrival time was 5105 minutes for the NORP and 263 minutes for the ORP groups, respectively. The mean estimated amount of organophosphate ingestion was 182 cc in ORP patients. Only 62 ORP patients reported alcohol intake, although most patients could not confirm a history of co-ingestion. There were no significant differences in diastolic blood pressure, heart rate, and body temperature between the poisoned groups. Systolic pressure was higher in the NORP group, and respiratory rate was higher in the ORP group. The initial mean GCS of the NORP group was significantly higher than that in the ORP group (13 vs. 10, p<0.001) (Table 2). Atropine and pralidoxime were administered to a total of 235 and 210 patients, respectively. The mean total dosages of atropine were 410 mg and 585 mg in the NORP and ORP groups, respectively. Among ORP patients, 190 received gastric lavage, and 143 were administered activated charcoal.
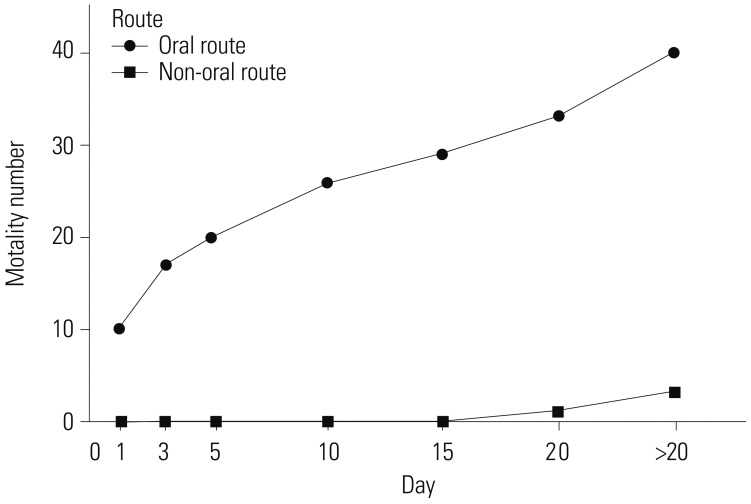
Overall, the incidence rates of respiratory complications were significantly lower in the NORP group. Moreover, the incidence of hypotension and CNS deficits during treatment were lower in the NORP group than in the ORP group. The final mortality rates were 8.8% and 24.3% in the NORP and ORP groups, respectively (Table 3). In the NORP group, two deaths occurred between 15 and 20 days, and one death occurred after 20 days. In the ORP group, 10 patients died within the first day, seven died between days 1 and 3 and another seven after 20 days (Fig. 1). The total lengths of hospitalization or ICU stay were shorter in the NORP group than in the ORP group. The APACHE II score was significantly lower in the NORP group than in the ORP group (Table 3).
Following 1:2 matching depending on the type of organophosphate, age, sex, systolic blood pressure, diastolic blood pressure, and pulse rate, there were no significant differences in demographics between the NORP and ORP groups (Tables 1, 2, 3). After matched analysis, all of the clinical complications, mortality rate and APACHE II score, were also significantly lower in the NORP group than in the ORP group.
Moreover, the laboratory findings did not significantly differ before and after matching analysis. Mean pH levels on initial arterial blood gas analysis were higher in the NORP group than in the ORP group. The initial laboratory values, including mean white blood cell count, mean serum lactate level, and levels of serum creatinine, alanine aminotransferase, and aspartate aminotransferase, were lower in the NORP group than in the ORP group (Table 3). The mean serum pseudocholinesterase level was maintained at a higher level in the NORP group than in the ORP group (Fig. 2).
After matching analysis, the relative risk ratio for mortality was 0.34-fold in the NORP group, compared to the ORP group. Relative risk analysis of NORP patients compared to ORP patients after matching yielded ratios for additional parameters as follows: ICU admission (0.61), applying endotracheal intubation (0.45), pulmonary edema (0.21), aspiration pneumonia (0.32), respiratory distress (0.47), hypotension (0.36), and CNS deficits (0.60) (Table 4). However, neither the total length of hospitalization nor the ICU stay was significantly shorter in the NORP group than in the ORP group (Table 3).
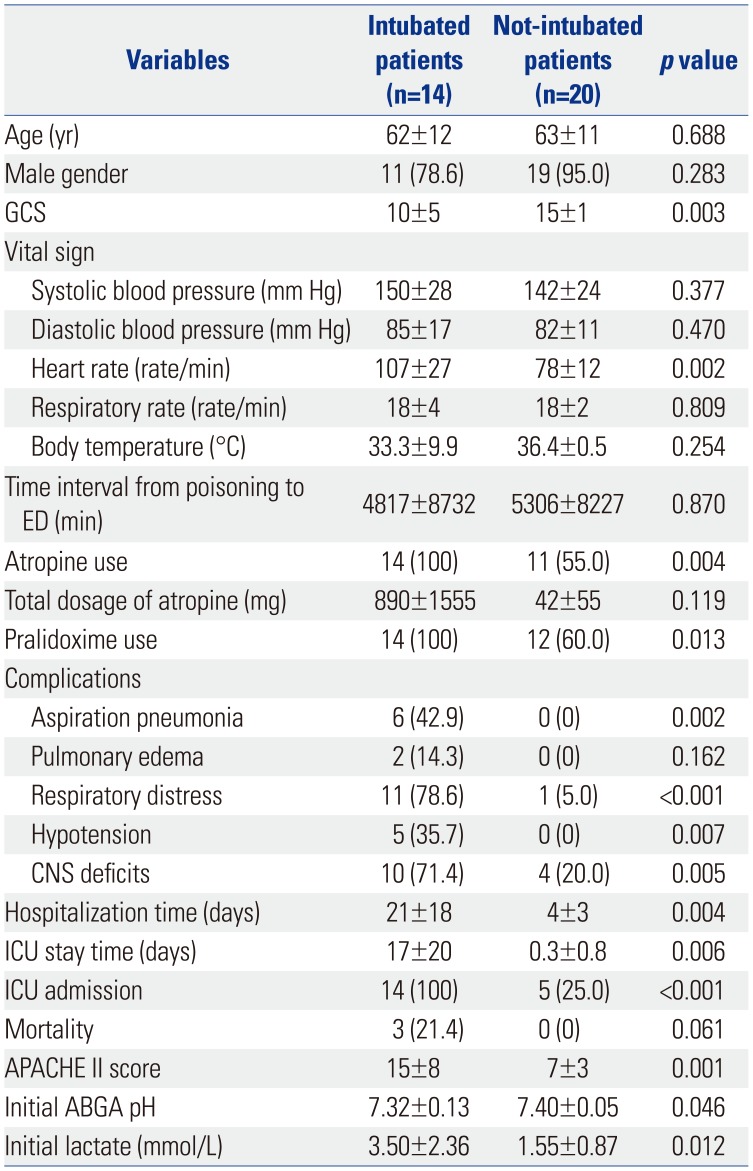
For subgroup analysis, 34 NORP patients were divided according to the need for endotracheal intubation. As one may predict, the intubated patients had more complications and worse laboratory results (Table 5).
In general, organophosphate poisoning is common in cases with suicidal intention.678 Past reports have discussed intentional organophosphate poisoning as an important clinical and public health problem in Asia.191011 This problem appears to be the same not only in developing countries, but also in rural areas in the Republic of Korea. Most of the intentional poisoning cases reviewed in this study occurred by ingestion through the oral route, while most of the unintentional poisoning cases occurred via the non-oral route. Therefore, clinicians could fail to notice the toxicity of organophosphate poisoning in NORP patients. However, we observed mortality in the cases of NORP, with many complications. Thus, our findings suggest that NORP is as critical as ORP. Moreover, although our results showed that the relative risk ratios for many of the clinical complications in the NORP group were smaller than those in the ORP group, the times spent on treatment were similar.
Recently, it was recognized that exposure to organophosphates is related with careless work management, including the lack of self-protection.1213 In this study, the majority of patients with NORP were not professional workers, but part-time workers, foreigners, or farmers. The majority of patients who visited the ED said that pesticide in a can sometimes leaked resulting in skin contact and that they did not use basic personal protective equipment, such as masks and glasses. Therefore, we suggest that workers must maintain and use thoroughly protective equipment.
The most serious complications due to organophosphate poisoning are respiratory complications.14 In the present study, 14 NORP patients required endotracheal intubation for the treatment of respiratory complications, indicating that emergency physicians should be aware of treatments for organophosphate poisoning regardless of the route of poisoning. NORP patients exhibited cholinergic symptoms such as dyspnea and vomiting after a significant delay, while unaware of their exposure to organophosphates. As a result, the mean time to ED visit in the NORP group was 5105 minutes, which was about 19 times longer than that for the patients who had attempted suicide.
The NORP group had a mean APACHE II score of 10 (predicted death rate: 15%), much lower than that of 20 (predicted death rate: 40%) in the ORP group.15 The actual mortality rates of 8.8% for the NORP group and 24.3% for the ORP group were not highly consistent with the predicted mortality rate on the APACHE II score. Three NORP patients arrived at the ED within 5 hours after exposure presenting with severe dizziness, vomiting, and dyspnea. All 3 patients were intubated and admitted to ICU. One of the patients, a 65-year old male, suffered acute respiratory failure after temporary cardiac arrest on the second day of hospitalization. He expired two weeks after the cardiac arrest. The other two patients, both males aged 67 and 75 years, suffered from septic shock with pneumonia during ICU care and expired within 3 weeks.
Serum cholinesterase levels have little prognostic value in patients with acute organophosphate poisoning, and these levels do not correlate with the amount of atropine required or the need for mechanical ventilation.1617 Therefore, clinicians should not rely only on pseudocholinesterase levels of patients suspected of organophosphate poisoning. In our study, the mean pseudocholinesterase level was significantly lower in the ORP group than that in the NORP group, and the recovery velocities of the mean pseudocholinesterase level were similar between the two groups. However, the time required for pseudocholinesterase level to return to near the normal range was shorter in the NORP group. In addition, despite the similar recovery velocity of pseudocholinesterase levels in both groups, it was difficult to judge patient prognosis or mortality only using an elevated pseudocholinesterase level.
The present study has several limitations. First, it is difficult to generalize the results of our study as it is a retrospective, single institutional study, and future studies involving multiple institutions are required to add to our findings. Second, there were differences in the treatment start time of the various organophosphate poisoned patients, making it difficult to generalize the final treatment results. Third, it was not possible to determine the identity of some of the pesticides that were the cause of the organophosphate poisoning. Fourth, it was not possible to measure the exact organophosphate exposure level causing NORP. Therefore, we could not confirm the differences in clinical results of poisoned patients according to the type of organophosphate pesticide.
In our study, the relative risk ratios for complications, including mortality, were lower in the NORP group than they were in the ORP group. However, the length of hospitalization or ICU stay did not differ significantly between the two groups. Therefore, medical observation and intensive therapeutic approaches are also needed for NORP regardless of the clinical severity thereof.
References
1. Jeyaratnam J. Acute pesticide poisoning: a major global health problem. World Health Stat Q. 1990; 43:139–144. PMID: 2238694.
2. Balali-Mood M, Balali-Mood K, Moodi M, Balali-Mood B. Health aspects of organophosphorous pesticides in asian countries. Iran J Public Health. 2012; 41:1–14.
3. Korean Rural Development Administration. The status of registred pesticide products. accessed on 2018 March 1. Available at: http://pis.rda.go.kr/registstus/agchmRegistStus/prdlstInqire.do.
4. Aygun D, Doganay Z, Altintop L, Guven H, Onar M, Deniz T, et al. Serum acetylcholinesterase and prognosis of acute organophosphate poisoning. J Toxicol Clin Toxicol. 2002; 40:903–910. PMID: 12507060.

5. Yang PY, Lin JL, Hall AH, Tsao TC, Chern MS. Acute ingestion poisoning with insecticide formulations containing the pyrethroid permethrin, xylene, and surfactant: a review of 48 cases. J Toxicol Clin Toxicol. 2002; 40:107–113. PMID: 12126181.

6. London L, Flisher AJ, Wesseling C, Mergler D, Kromhout H. Suicide and exposure to organophosphate insecticides: cause or effect? Am J Ind Med. 2005; 47:308–321. PMID: 15776467.

7. Yurumez Y, Durukan P, Yavuz Y, Ikizceli I, Avsarogullari L, Ozkan S, et al. Acute organophosphate poisoning in university hospital emergency room patients. Intern Med. 2007; 46:965–969. PMID: 17603234.

8. Tsai JR, Sheu CC, Cheng MH, Hung JY, Wang CS, Chong IW, et al. Organophosphate poisoning: 10 years of experience in southern Taiwan. Kaohsiung J Med Sci. 2007; 23:112–119. PMID: 17389175.

9. Van der Hoek W, Konradsen F, Athukorala K, Wanigadewa T. Pesticide poisoning: a major health problem in Sri Lanka. Soc Sci Med. 1998; 46:495–504. PMID: 9460829.

11. World Health Organization. The world health report 2002: reducing risks, promoting healthy life. Geneva, Switzerland: World Health Organization;2002.
12. Liang Y, Tong F, Zhang L, Li W, Huang W, Zhou Y. Fatal poisoning by terbufos following occupational exposure. Clin Toxicol (Phila). 2018; 56:140–142. PMID: 28681657.

13. Rubio CR, Felipe Fernández C, Manzanedo Bueno R, Del Pozo BA, García JM. Acute renal failure due to the inhalation of organophosphates: successful treatment with haemodialysis. Clin Kidney J. 2012; 5:582–583. PMID: 26069807.

14. Wang CY, Wu CL, Tsan YT, Hsu JY, Hung DZ, Wang CH. Early onset pneumonia in patients with cholinesterase inhibitor poisoning. Respirology. 2010; 15:961–968. PMID: 20663095.

15. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985; 13:818–829. PMID: 3928249.
16. Nouira S, Abroug F, Elatrous S, Boujdaria R, Bouchoucha S. Prognostic value of serum cholinesterase in organophosphate poisoning. Chest. 1994; 106:1811–1814. PMID: 7988206.

17. El-Naggar AER, Abdalla MS, El-Sebaey AS, Badawy SM. Clinical findings and cholinesterase levels in children of organophosphates and carbamates poisoning. Eur J Pediatr. 2009; 168:951–956. PMID: 18998164.

Fig. 2
Repeated measured mean values of serum pseudocholinesterase in non-oral route poisoning vs. oral route poisoning.
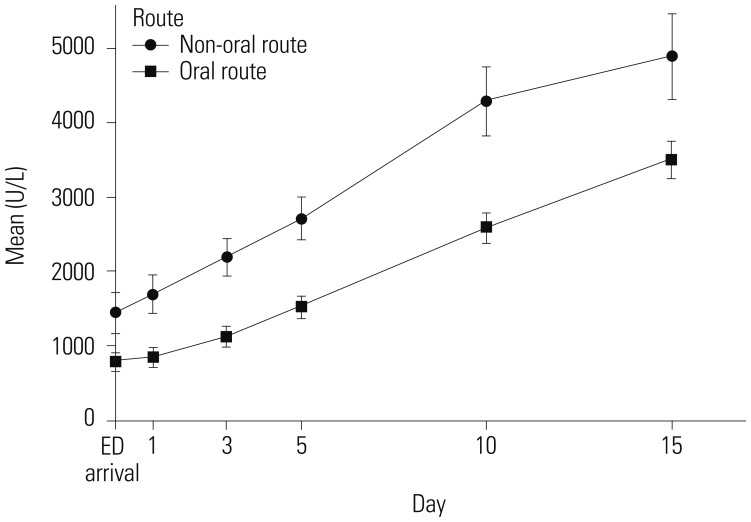
Table 1
Major Organophosphate Components to Which Patients Were Exposed Before and After 1:2 Propensity Score Matching
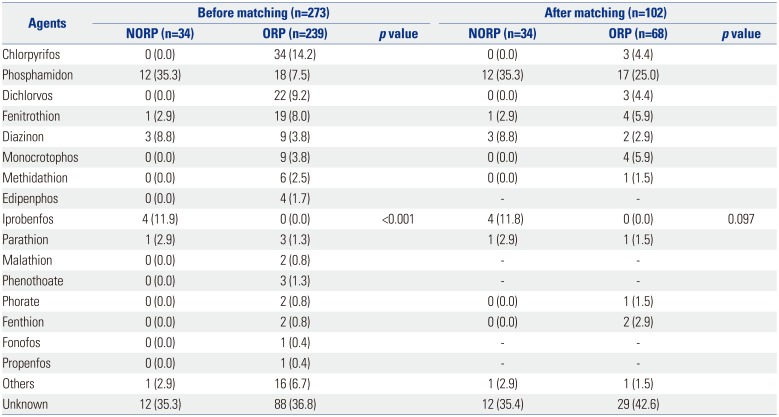
Table 2
Demographics of All Organophosphate Poisoning Patients Before and After 1:2 Propensity Score Matching
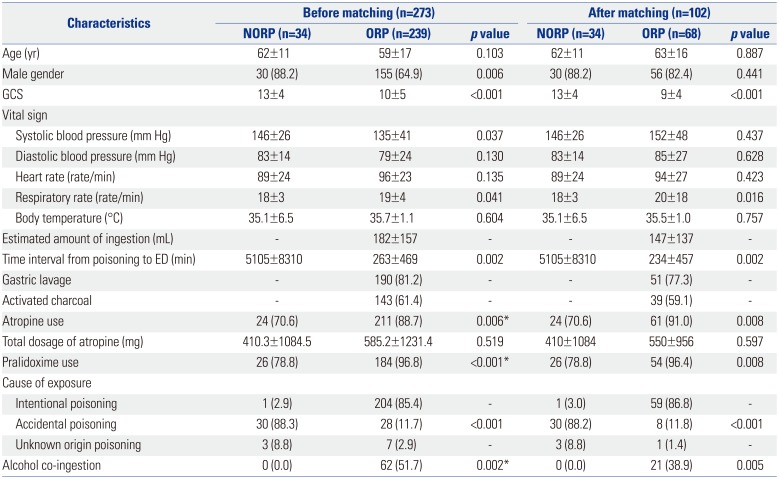
Table 3
Complications and Laboratory Values of All Organophosphate Poisoning Patients Before and After 1:2 Propensity Score Matching
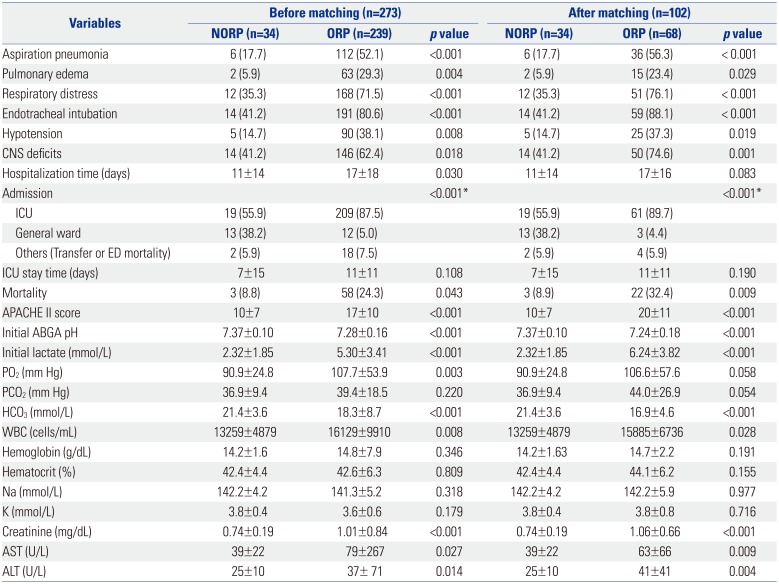
NORP, non-oral route poisoning; ORP, oral route poisoning; CNS, central nervous system; ICU, intensive care unit; ED, emergency department; APACHE II, Acute Physiology and Chronic Health Evaluation II; ABGA, arterial blood gas analysis; WBC, white blood cell; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Data are expressed as number (%) or mean±SD.
*Fisher's exact test.
Table 4
Relative Risk Analysis of Non-Oral Route Poisoning Patients Compared to Those of Oral Route Poisoning Patients after Matching
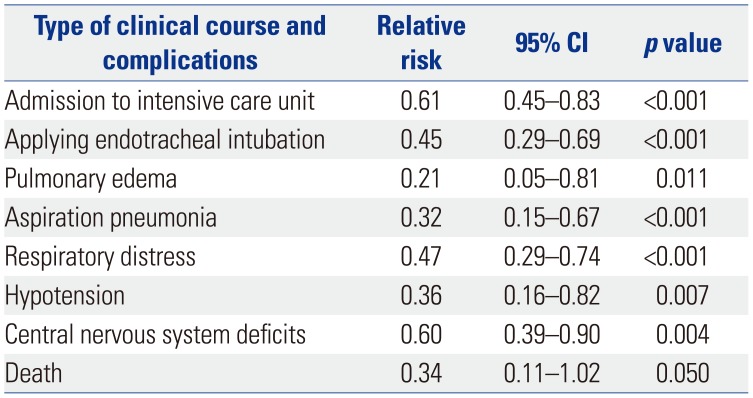
Table 5
Demographics and Clinical Characteristics of 34 Non-Oral Route Poisoning Patients according to Endotracheal Intubation




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download