Abstract
Purpose
Cefaclor, a second-generation oral cephalosporin, is known to cause IgE-mediated hypersensitivity. Assays of serum-specific IgE (sIgE) to cefaclor are commercially available via the ImmunoCAP system (Thermo Fisher Scientific). While serum levels of sIgE >0.35 kU/L are considered indicative of an allergy, some patients with cefaclor allergy show low serum IgE levels. This study aimed to evaluate the proper cut-off levels of sIgE in the diagnosis of immediate hypersensitivity to cefaclor.
Materials and Methods
A total of 269 patients with drug allergy history, who underwent assays of sIgE to cefaclor at Ajou University hospital and Dong-A University Hospital, were reviewed retrospectively. Among them, 193 patients exhibited cefaclor-induced immediate hypersensitivity with certain or probable causality of an adverse drug reaction according to the WHO-UMC (the World Health Organization-the Uppsala Monitoring Centre) algorithm, and 76 controls showed delayed hypersensitivity reactions to non-antibiotics.
Results
In total, 126 of the 193 patients (65.3%) experienced anaphylaxis; they had higher serum sIgE levels than patients with immediate hypersensitivity who did not experience anaphylaxis (6.36±12.39 kU/L vs. 4.28±13.61 kU/L, p<0.001). The best cut-off value for cefaclor-induced immediate hypersensitivity was 0.11 kU/L, with sensitivity of 80.2% and specificity of 81.6%. A cut-off value of 0.44 kU/L showed the best sensitivity (75.4%) and specificity (65.7%) for differentiating anaphylaxis from immediate hypersensitivity reactions.
Cefaclor, a second-generation oral cephalosporin, is used for a wide range of bacterial infections. Reports have indicated that cefaclor induces various kinds of reactions, such as erythematous or popular eruptions, urticaria, serum sickness-like reactions, hypersensitivity myocarditis, and anaphylaxis, in both adults and children.12345 Immediate hypersensitivity reactions to cephalosporins are common, and they are usually IgE-mediated reactions. Anaphylaxis, in particular, is the most commonly reported immediate allergic reaction to cefaclor.
Skin testing and measurement of serum-specific IgE (sIgE) levels to cefaclor have been well documented.3567 Skin testing is the primary diagnostic method, although, in patients with a history of severe reactions, in vitro tests may be recommended.
Additionally, sIgE to cefaclor can be measured by the commercially available ImmunoCAP system (Thermo Fisher Scientific, Uppsala, Sweden), with high detection rates (80–90%).57 Serum sIgE levels >0.35 kU/L against allergens measured by ImmunoCAP are regarded as indicating positivity to allergen, regardless of type, such as foods, inhalants, and drugs, and clinical manifestations, including urticarial, skin rash, and anaphylaxis. However, in real practice, some patients with hypersensitivity to cefaclor show low IgE levels below 0.35 kU/L. In this study, we evaluated proper cut-off levels of serum sIgE to cefaclor for diagnosis of cefaclor-induced immediate hypersensitivity and anaphylaxis.
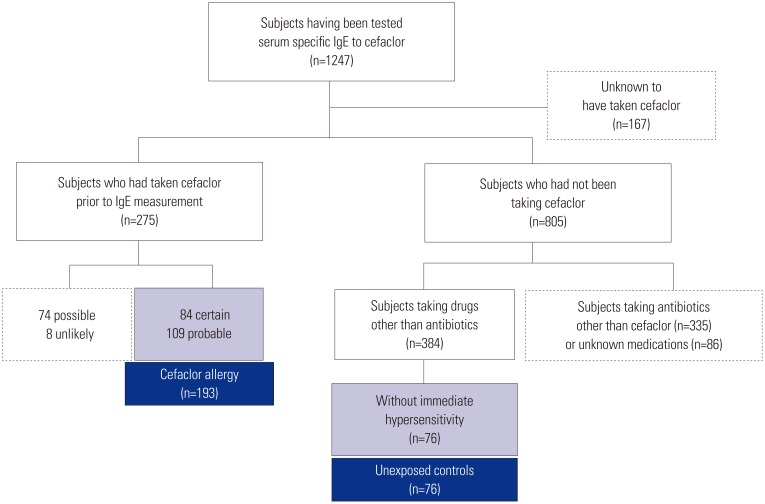
We reviewed a total of 1247 patients who had undergone measurement of serum sIgE levels to cefaclor in pharmacovigilance centers at Ajou and Dong-A University hospitals from June 2002 to March 2016 (Fig. 1). Among them, 805 patients had not been prescribed cefaclor; 275 were identified as taking cefaclor prior to sIgE measurement; and 167 patients had no information on cefaclor prescription before undergoing measurement of sIgE to cefaclor. We excluded the 167 patients lacking information on cefaclor prescription.
Based on causality assessment using WHO-UMC (the World Health Organization–the Uppsala Monitoring Centre) criteria, 84 certain, 109 probable, 74 possible, and eight unlikely cases were identified from 275 patients reported as having cefaclor allergy. The causality of “certain” is based on the condition that adverse drug reaction (ADR) is confirmed by provocation test or occurs repeatedly when the causative medication is reexposed. In addition, when ADR occurs after taking another drug at the same time and if the other drug can be excluded by skin tests or provocation tests, we can deem the suspected medication as a certain cause. Of these 275 patients, 193 who were diagnosed with cefaclor-induced immediate hypersensitivity with certain or probable causality were included in the present study. Among the 805 patients who had not been prescribed cefaclor, we defined 76 subjects with non-immediate hypersensitivity reactions to other medications, except antibiotics, who had undergone measurement of serum sIgE levels to cefaclor during the study period as unexposed controls. Subjects who had been prescribed antibiotics other than cefaclor or unknown medications were also excluded. This study was approved by the institutional review boards of both hospitals (AJIRB-MED-MDB-16-166).
All subjects with cefaclor allergy and the included controls underwent measurement of serum sIgE levels to cefaclor using ImmunoCAP® (Thermo Fisher Scientific). We also collected the results of serum sIgE to penicilloyl G and V, ampicillin, and amoxicillin, as they were available.
Quantitative and qualitative results are given as means±SD and absolute numbers or frequencies, respectively. Descriptive statistics were performed using SPSS software ver. 19.0 (IBM Corp., Armonk, NY, USA). Kolmogorov-Smirnov test revealed that total and sIgE levels in the present study were not distributed normally (both p values <0.001). Statistical significance was assessed using Mann-Whitney U-test for continuous variables and Fisher's exact test for categorical variables. Correlation between serum sIgE levels to cefaclor and IgEs to penicillin, ampicillin, and amoxicillin was analyzed using Spearman's rho. p values <0.05 were considered to indicate statistical significance. Receiver-operating characteristic (ROC) curves were drawn to determine the optimal cut-off values of serum sIgE to cefaclor levels through which to identify immediate hypersensitivity reactions to cefaclor, and to discriminate cefaclor-induced anaphylaxis. Therefrom, the area under the curve (AUC) with a 95% confidence interval (CI) was estimated.
Mean ages and gender differences were not noted between cefaclor hypersensitivty and unexposed control groups (Table 1). Among combined allergic diseases, the frequencies of asthma, allergic rhinitis, and chronic urticaria were similar between the two groups. However, drug allergy was more commonly identified in unexposed control group than in cefaclor hypersensitivity group (18.4% vs. 2.6%, p<0.001). Immediate hypersensitivity and anaphylaxis were identified in 172 and 126 patients with cefaclor hypersensicitivity, respectively. Of the 126 patients with anaphylaxis, 12 (9.5%), 76 (60.3%), and 38 (30.2%) patients were identified with mild, moderate, and severe anaphylaxis, respectively. Log-transformed serum total IgE and sIgE levels to cefaclor were significantly higher in cefaclor hypersensitivity group than in unexposed control group (2.3±0.6 kU/L vs. 2.1±0.62 kU/L, p=0.011 for total IgE; 5.3±12.2 kU/L vs. 0.2±0.93 kU/L, p<0.001 for sIgE to cefaclor). The mean interval between onset of adverse reactions and sIgE measurement was 13.4±15.9 days (minimum to maximum, 0–67) in patients with cefaclor hypersensitivity.
Among patients with cefaclor hypersensitivity, serum sIgE levels to cefaclor were significantly different between cases reported as immediate and those reported as non-immediate hypersensitivity (5.80±12.72 kU/L vs. 1.30±4.40 kU/L, p<0.001) (Fig. 2). The mean level of serum sIgE to cefaclor was significantly higher in patients with anaphylaxis than in those with other immediate reactions (6.36±12.39 kU/L vs. 4.28±13.61 kU/L, p<0.001) (Fig. 2). However, there was no significant difference in the mean levels of sIgE to cefaclor among three subgroups of anaphylaxis, based on the clinical severity thereof (5.69±6.39 kU/L in mild vs. 6.23±13.70 kU/L in moderate vs. 6.82±11.19 kU/L in severe anaphylaxis, p value >0.05). In patients with cefaclor-induced immediate hypersensitivity, sIgE to cefaclor was significatly correlated with total IgE (Spearman's rho 0.248, p=0.002) and sIgE levels to penicylloyl G (0.198, p=0.041) and amoxicilloyl (0.313, p<0.001). However, no significant correlations were observed between sIgE to cefaclor and IgE to penicilloyl V (−0.082, p=0.406) and ampicilloyl (−0.069, p=0.419).
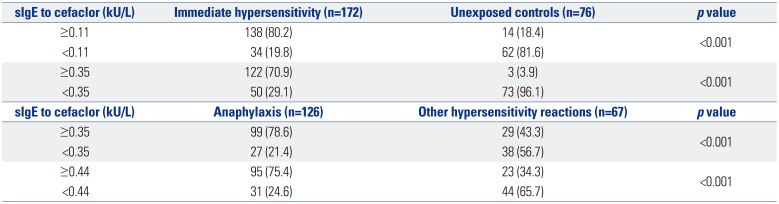
ROC curve analysis yielded a value of 0.11 kU/L of serum sIgE to cefaclor as the optimal cut-off for discriminating immediate hypersensitivity to cefaclor in patients with cefaclor hypersensitivity of certain or probable causality (AUC 0.874, 95% CI 0.829–0.919, p<0.001). Using a serum sIgE level ≥0.11 kU/L as the cut-off value, the sensitivity and specificity for detecting immediate hypersensitivity to cefaclor were 80.2% and 81.6%, respectively (Table 2). When we applied a cut-off of 0.35 kU/L, the sensitivity and specificity for confirming immediate hypersensitivity to cefaclor were 70.9% and 71.4%, respectively. However, there was no statistically significant difference in AUCs between two ROC curves with 0.11 kU/L and 0.35 kU/L for determining immediate hypersensitivity (p=0.521) (Fig. 3A).
For detecting anaphylaxis among patients with cefaclor hypersensitivity, a cut-off of 0.44 kU/L was identified (AUC 0.726, 95% CI 0.648–0.804, p<0.001). With a serum sIgE level ≥0.44 kU/L as the cut-off value, the sensitivity and specificity for detecting cefaclor-induced anaphylaxis were 75.4% and 65.7%, respectively (Table 2). A cut-off of 0.35 kU/L of serum sIgE to cefaclor showed sensitivity of 78.6% and specificity of 56.7% to discrimate anaphylaxis among cefaclor-induced hypersensitivity reactions. However, no significant difference in comparison of two ROC curves using sIgEs of 0.44 kU/L and 0.35 kU/L to detect cefaclor-induced anaphylaxis (p=0.617) (Fig. 3B).
The prevalence of allergies to antibiotics has been reported to be 5–10% worldwide.89 However, a considerable amount of patients with suspected antibiotics allergy are not confirmed by an appropriate diagnostic workup: the diagnosis of antibiotics allergy is usually based on clinical history. Although skin tests have been used for the diagnosis of hypersensitivity reactions against antibiotics, some cephalosporins, such as cefaclor, are not available in injectable form, and intradermal tests are not possible.10 Moreover, although provocation tests are regarded as the gold standard to confirm drug allergy, they are not recommended in patients who have experienced life-threatening reactions, including severe anaphylaxis. Meanwhile, previous studies have demonstrated that immunoassay to measure sIgE antibodies to betalactams show good aggreement with skin testing.811 Compared with a lower sensitivity (36.2%) for detecting serum sIgE to cefaclor-albumin conjugate by homemade ELISA, ImmunoCAP has been found to show a higher sensitivity (89.4%) for detecting cefaclor allergy at a cut-off value of 0.35 kU/L.7
Using data from two pharmacovigilance centers in Korea, the present study suggests that a cut-off value of 0.44 kU/L of serum sIgE measured by ImmunoCAP can diagnose cefaclor-induced anaphylaxis among patients with immediate hypersensitivity to cefaclor. Compared to using 0.35 kU/L, the specificity for determining cefaclor-induced anaphylaxis was higher when we applied 0.44 kU/L as a cut-off value. Since cefaclor is an oral antibiotic with broad-spectrum use for urinary tract infections, skin infections, otitis media, and others, it has been frequently prescribed in patients of all ages. It is well known that adverse reactions associated with cefaclor are mostly immediate hypersensitivity, and anaphylaxis is the most common phenotype of cefaclor allergy.4712 In the present study, among 193 cases of cefaclor allergy of certain or probable causality, 126 (65.3%) involved anaphylaxis.
Most patients with suspected cefaclor allergy are also exposed to antipyretics or analgesics, which are the most common culprits for immediate drug hypersensitivity, and both skin tests and immunoassay are not applicable for most patients with nonsteroidal anti-inflammatory drug (NSAID) hypersensitivity. Therefore, screening for the possibility of cefaclor-induced hypersensitivity among patients who experience urticaria, angioedema, and anaphylaxis during treatment with cefaclor and NSAIDs is helpful for physicians in determining the culprit. In this study, a cut-off value of 0.11 kU/L (80.2%) of serum sIgE to cefaclor showed greater sensitivity in differentiating patients with cefaclor-induced immediate hypersensitivity than 0.35 kU/L (70.9%). With the criterion of 0.11 kU/L, however, two patients with non-immediate hypersensitivity to cefaclor were misdiagnosed as cefaclor-induced immediate hypersensitivity. Notwithstanding, 16 (9.3%) patients with cefaclor allergy whose serum sIgE levels to cefaclor were between 0.11 kU/L and 0.35 kU/L could avoid rechallenge of cefaclor by being diagnosed with cefaclor allergy, based on immunoassay results. For patients experiencing immediate hypersensitivity upon being exposed to cefaclor and NSAIDs simultaneously, clinicians can consider performing oral provocation test with NSAIDs to confirm the diagnosis when serum sIgE levels to cefaclor are <0.11 kU/L. Nevertheless, it has been reported that cefaclor-induced anaphylaxis can develop in a subset of patients by non-IgE mediated mechanisms, such as direct activation of basophils or mast cells.713 Accordingly, even in patients with sIgE to cefaclor <0.11 kU/L, but who are still suspected as having cefaclor allergy, clinicians may seek to carefully perform oral provocation with cefaclor.
Cephalosporins allergy is dependent on antigenic determinants for each patient. Sensitization to specific cephalosporin haptens, or to common structures shared with penicillins or other cephalosporins, is possible.1415 Due to identical R1 side chains, cross-reactivity between cefaclor and cephalexin has been described in a previous study.16 However, cross-reactivity related to R2 side chain16 and its metabolite pyrazinone hapten has also been noted.17 Venemalm17 reported that, in nine of 15 patients who were positive to cefaclor ImmunoCAP, sIgE to pyrazinone hapten was detected. Moreover, these 15 patients were also positive to at least one of penicilloyl G, penicilloyl V, ampicilloyl, and amoxicilloyl. They suggested pyrazinone hapten as a common antigenic determinant in a subgroup of patients with allergy to cefaclor and penicillins.17 A recent study reported that two-thirds of cases with cephalosporin allergy showed a selective recognition of R1 side chain, and the other one-third had crossreactivity with different cephalosporins.6 While cefaclor shares the same R1 side chain with ampicillin, crossreactivity with penicillin and amoxicillin has also been reported in patients with cefaclor allergy.671618 Indeed, significant correlations for sIgE to cefaclor with total IgE and sIgE to amoxicilloyl and penicillinoyl G, but not with ampicilloyl or penicilloyl V, were observed in the present study. Meanwhile, allergic reaction to β-lactams is determined by the chemical structures of metabolites, as well as by the nature of conjugates and presentation by antigen-presenting cells.19 Moreover, non-IgE mediated immediate hypersensitivity reactions to cefaclor have already been proven.7 Therefore, the potential of crossreactivity between cefaclor and other betalactams cannot be simply determined.
The present study had some limitations. Although cefaclor hypersensitivity cases deemed certain or probable in causality were included in this study, positive predictive values of our cut-off levels of cefaclor sIgE could not be determined since it was a retrospective study based on a spontaneously reported pharmacovigilance system. Conversely, we also cannot assert that all patients included in the unexposed controls of this study had never taken cefaclor before. Moreover, the time interval between onset of cefaclor allergy and measurement of sIgE to cefaclor was not controlled in the present study. Previous studies have shown that the possibility of sIgE detection in skin or serum decreases as the time interval between reaction and measurement increases.20 However, there has been a report of one patient whose sIgE to cefaclor persisted for at least 6 years after reactions.21 Hidden contact with cefaclor or exposure to other antibiotics sharing common allergenic peptides with cefaclor, as well as reactivity to several penicillin determinants, has been suggested as potential factors to induce persistent presence of sIgE.2021 Indeed, the cases in the present study involved a relatively short time period with which to measure sIgE to cefaclor after adverse reactions at a mean of 13.4 days and a maximum of 67 days.
Different weights can be applied to the sensitivity or specificity of a test to measure sIgE antibodies, as clinical manifestations and causes of drug allergy can vary. With the correct sIgE criteria to differentiate clinical phenotypes of cefaclor hypersensitivity, unwanted provocation tests can be avoided in cases of severe manifestation. In conclusion, the present study indicates that 0.11 kU/L and 0.44 kU/L of serum sIgE to cefaclor might be proper levels for detecting immediate hypersensitivity and anaphylaxis caused by cefaclor, respectively. However, prospective studies to investigate the negative and positive preditive values of the suggested cut-off values of cefaclor sIgE antibodies will be required to confirm the clinical performance of these criteria.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI17C0970) and a grant from the Ministry of Food and Drug Safety and the Korean Institute of Drug Safety and Risk Management for operation of the regional pharmacovigilance center in 2018.
References
1. Beghetti M, Wilson GJ, Bohn D, Benson L. Hypersensitivity myocarditis caused by an allergic reaction to cefaclor. J Pediatr. 1998; 132:172–173. PMID: 9470025.

2. Kearns GL, Wheeler JG, Rieder MJ, Reid J. Serum sickness-like reaction to cefaclor: lack of in vitro cross-reactivity with loracarbef. Clin Pharmacol Ther. 1998; 63:686–693. PMID: 9663184.

3. King BA, Geelhoed GC. Adverse skin and joint reactions associated with oral antibiotics in children: the role of cefaclor in serum sickness-like reactions. J Paediatr Child Health. 2003; 39:677–681. PMID: 14629499.

4. Novembre E, Mori F, Pucci N, Bernardini R, Romano A. Cefaclor anaphylaxis in children. Allergy. 2009; 64:1233–1235. PMID: 19385950.

5. Nam YH, Kim JE, Hwang EK, Jin HJ, Shin YS, Ye YM, et al. Clinical and immunologic evaluations of immediate hypersensitivity to cefaclor. Korean J Asthma Allergy Clin Immunol. 2011; 31:192–198.
6. Atanasković-Marković M, Velicković TC, Gavrović-Jankulović M, Vucković O, Nestorović B. Immediate allergic reactions to cephalosporins and penicillins and their cross-reactivity in children. Pediatr Allergy Immunol. 2005; 16:341–347. PMID: 15943598.

7. Yoo HS, Kim SH, Kwon HS, Kim TB, Nam YH, Ye YM, et al. Immunologic evaluation of immediate hypersensitivity to cefaclor. Yonsei Med J. 2014; 55:1473–1483. PMID: 25323882.

8. Doña I, Torres MJ, Montañez MI, Fernández TD. In vitro diagnostic testing for antibiotic allergy. Allergy Asthma Immunol Res. 2017; 9:288–298. PMID: 28497915.

9. Gomes ER, Demoly P. Epidemiology of hypersensitivity drug reactions. Curr Opin Allergy Clin Immunol. 2005; 5:309–316. PMID: 15985812.

10. Har D, Solensky R. Penicillin and beta-lactam hypersensitivity. Immunol Allergy Clin North Am. 2017; 37:643–662. PMID: 28965632.

11. Antunez C, Blanca-Lopez N, Torres MJ, Mayorga C, Perez-Inestrosa E, Montañez MI, et al. Immediate allergic reactions to cephalosporins: evaluation of cross-reactivity with a panel of penicillins and cephalosporins. J Allergy Clin Immunol. 2006; 117:404–410. PMID: 16461141.

12. Romano A, Guéant-Rodriguez RM, Viola M, Amoghly F, Gaeta F, Nicolas JP, et al. Diagnosing immediate reactions to cephalosporins. Clin Exp Allergy. 2005; 35:1234–1242. PMID: 16164453.

13. Pumphrey RS, Davis S. Under-reporting of antibiotic anaphylaxis may put patients at risk. Lancet. 1999; 353:1157–1158.

14. Pham NH, Baldo BA. beta-Lactam drug allergens: fine structural recognition patterns of cephalosporin-reactive IgE antibodies. J Mol Recognit. 1996; 9:287–296. PMID: 9131470.
15. Baldo BA. Penicillins and cephalosporins as allergens--structural aspects of recognition and cross-reactions. Clin Exp Allergy. 1999; 29:744–749. PMID: 10336588.
16. Romano A, Gaeta F, Valluzzi RL, Maggioletti M, Zaffiro A, Caruso C, et al. IgE-mediated hypersensitivity to cephalosporins: cross-reactivity and tolerability of alternative cephalosporins. J Allergy Clin Immunol. 2015; 136:685–691.e3. PMID: 25930196.

17. Venemalm L. Pyrazinone conjugates as potential cephalosporin allergens. Bioorg Med Chem Lett. 2001; 11:1869–1870. PMID: 11459649.

18. Pichichero ME, Zagursky R. Penicillin and cephalosporin allergy. Ann Allergy Asthma Immunol. 2014; 112:404–412. PMID: 24767695.

19. Ariza A, Mayorga C, Fernandez TD, Barbero N, Martín-Serrano A, Pérez-Sala D, et al. Hypersensitivity reactions to β-lactams: relevance of hapten-protein conjugates. J Investig Allergol Clin Immunol. 2015; 25:12–25.
20. Blanca M, Torres MJ, García JJ, Romano A, Mayorga C, de Ramon E, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol. 1999; 103(5 Pt 1):918–924. PMID: 10329829.
21. Bernardini R, Novembre E, Lombardi E, Pucci N, Rossi ME, Vierucci A. Long persistence of IgE antibody to cefaclor. Allergy. 2000; 55:984–985. PMID: 11030389.

Fig. 2
Levels of serum sIgE to cefaclor according to clinical phenotypes of cefaclor hypersensitivity. sIgE, specific IgE.

Fig. 3
ROC curves for determining immediate hypersensitivity to cefaclor (A) and discriminating anaphylaxis among patiens with cefaclor hypersensitivity (B). sIgE, specific IgE; CI, confidence interval; AUC, area under the curve; ROC, receiver operating characteristic.
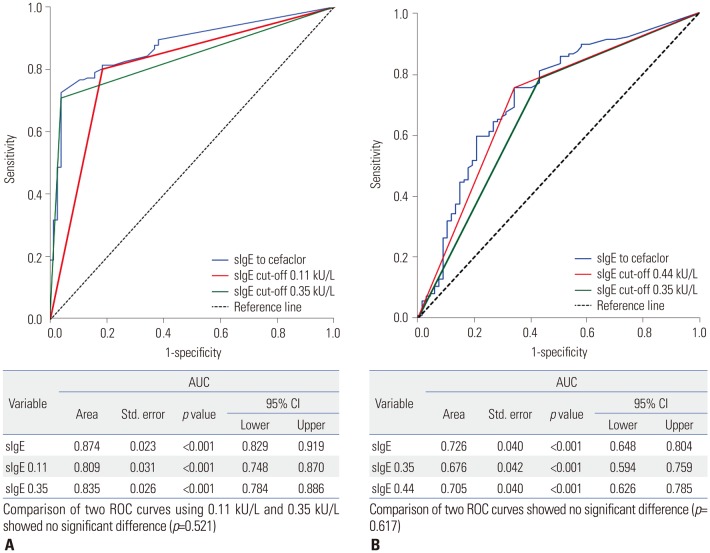
Table 1
Clinical Characteristics of Study Subjects
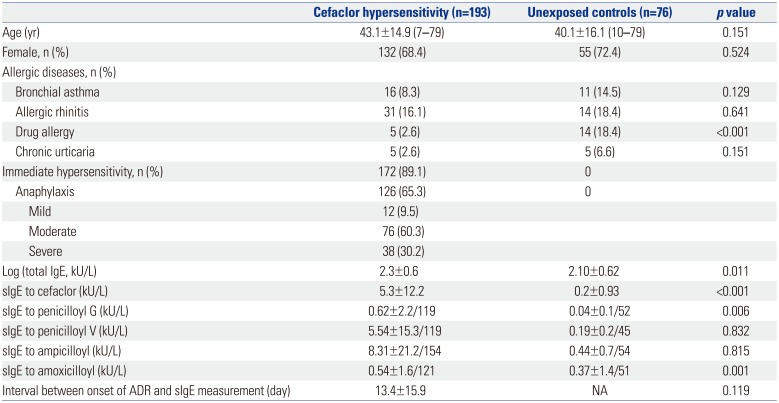
Table 2
Cut-off Values of sIgE to Cefaclor for Detecting Immediate Hypersensitivity and Anaphylaxis




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download