Abstract
Purpose
This study aimed to screen for differentially expressed microRNAs (miRNAs) in the colons of rats with visceral hypersensitivity to build the expression profiles of miRNAs therein and to determine the mechanism of Tongxieyaofang use in the treatment of irritable bowel syndrome (IBS).
Materials and Methods
Forty Sprague-Dawley rats were divided randomly into four groups: control group, model control group (induced by rectum stimulus and evaluated by abdominal withdraw reaction), treatment control group (normal saline), and Tongxieyaofang group (treated with Tongxieyaofang). We screened for differential expression of colonic mucosal miRNAs using liquid chip technology and verified the expression thereof using reverse transcription-PCR.
Results
The visceral hypersensitivity rat model was successfully established. We found the expression of let-7f, let-7i, miR-130b, miR-29a, miR-132, miR-21, and miR-375 to be up-regulated (p<0.05), while the expression of miR-24, miR-31a, miR-192, miR-221, and miR-223 was down-regulated (p<0.05) in the visceral hypersensitivity rats. After treatment with Tongxieyaofang, the expression of let-7f, let-7i, miR-130b, miR-29a, miR-132, miR-21, and miR-375 was reduced (p<0.05), whereas the expression of miR-24, miR-31a, miR-192, miR-221, miR-223 was increased, compared to the treatment control group (p<0.05).
Irritable bowel syndrome (IBS) is characterized by chronic abdominal pain and an alteration in bowel habits (diarrhea and/or constipation).1 It is one of the most frequent digestive tract disorders encountered by general practitioners and gastroenterologists. The underlying pathophysiological mechanisms of abdominal pain and chronic symptoms in IBS are still unclear.2 There are a number of factors involved therein, including the enteric nervous system, inflammatory processes, diet, and microbiota. Some patients with diarrhea-predominant IBS exhibit increased intestinal permeability or low-grade inflammation.34 Some patients with IBS experience enhanced responses to distension of the gut lumen or visceral hypersensitivity, accounting for symptoms of urgency, bloating, and abdominal pain.5 In response to a triggering event, such as inflammation, trauma, or environmental stress, the primary visceral afferents to the gut become sensitized, leading to chronic visceral pain in IBS patients.6 Although visceral hypersensitivity is a major cause of IBS, the mechanism of visceral hypersensitivity in IBS is still unclear.
MicroRNAs (miRNAs) were first identified in 1993 as small, endogenous, and approximately 21–23 nucleotide long non-coding RNAs that had the capacity for gene regulation after transcription.7 MiRNAs have emerged as regulators involved in biological functions, such as cellular development, differentiation, proliferation, apoptosis, and metabolism, and were shown to take part in a number of chronic disease processes, including gastrointestinal disorders.8 Research has shown unique miRNA present both in circulating blood microvesicles and colonic biopsies of IBS patients with diarrhea and increased intestinal permeability.9 Another study10 showed that miR-24 plays a role in the pathogenesis of IBS by regulating SERT expression. Hsa-miR-150 has been reported to be associated with inflammatory bowel disorders and pain and to interact with a protein kinase and inflammatory pathways. Hsa-miR-342-3p was reported to interact with mRNAs involved in pain signaling, colonic motility, and smooth muscle function.11 Therefore, miRNAs appear to play an important role in IBS. However, studies in the literature have only involved one or two miRNAs. Through literature search, we gathered statistics about differentially expressed miRNAs in intestinal mucosal tissues and cells in regards to intestinal mucosal barrier injury, intestinal inflammation, gastrointestinal cancer, and inflammatory bowel disease. Therefrom, we choose 21 miRNAs in rats as study targets: let-7f, let-7i, miR-21, miR-23a, miR-23b, miR-24, miR-29a, miR-31a, miR-122-5p, miR-130b, miR-132, miR-143, miR-145, miR-146a, miR-192, miR-199a, miR-200b, miR-217, miR-221, miR-223, and miR-375.61213141516171819202122
Tongxieyaofang, a classical prescription in traditional Chinese medicine, is composed of white atractylodes rhizome, white peony root, dried old orange peel, and Ledebouriella root. It has been widely used in the treatment of diarrhea. However, the complete mechanism of the use of Tongxieyaofang in the treatment of IBS has not been fully described.
The purpose of this study was to screen the different expression of miRNAs in the colons of rats using liquid chip technology and reverse transcription-PCR and to build expression profiles of colonic mucosal miRNA in visceral hypersensitivity in rats. Additionally, changes of the expression of miRNAs were evaluated to determine the mechanism of action for Tongxieyaofang in the treatment of IBS.
Forty adult female Sprague-Dawley rats, weighing 210±10 g, were provided by the Animal Experiment Center of Zhejiang Traditional Chinese Medical University (Hangzhou, China). The rats were fed in the experimental animal center (24±2℃; humidity, 55±5%; noise <50 dB; and light/dark cycle, 12/12 hours). The rats were divided into four groups, with 10 rats in each group: control group, model group, treatment control group (normal saline), and Tongxieyaofang group.
The rats were fed normally for 2 weeks, and then, we observed their state and evaluated their visceral sensitivity by abdominal withdraw reaction.
Camphor ball odor was used as a conditioned stimulus while maintaining a rectal distention pressure >60 mm Hg (1 mm Hg=0.133 kPa); extremities constraint was used as unconditioned stimulus. The extremities and trunk of the rats were fixed for 45 min in the camphor ball cage. Meanwhile, a catheter was inserted into the rectum of rats, and there was about 1 cm from the distal end of the air bladder to anus. We fixed the catheter at the root of each rat's tail. Rectal dilatation was performed by intermittent aeration with 1.6 mL (hydrostatic pressure >60 mm Hg) for 60 seconds, spaced 3 minutes apart, and repeated 10 times. This stress process was performed on the first, second, and fifth day. On the fourth, sixth, and eighth day, the conditioned stimulus was performed. The rats were fed normally on the third and seventh day. Then, we evaluated visceral sensitivity by abdominal withdraw reaction.
We evaluated the visceral sensitivity by abdominal withdraw reaction. To do so, an 8F urethral catheter lubricated by liquid paraffin was inserted through the rectum. There was about 1 cm from the distal end of the air bladder to anus, and the urethral catheter was fixed at the root of the tail. The rats were placed on the platform, and after they adapted to the environment, we gradually injected water into the sacrum and recorded the amount of water injected when the rats lifted their abdomen and bent their back. Rectal dilatation lasted for 30 seconds at a time and was repeated three times. The average of the three value was recorded.
A part of the colon was removed and placed in oxygenated Tyrol's solution after laparotomy incision. Then, a 2-cm segment of the colon was placed in a 10-mL vessel containing Tyrode's solution, which was filled with 95% O2 and 5% CO2, and maintained at temperature of 37℃.
According to published studies, the 21 miRNAs we tested were confirmed to be related to intestinal disease, including miR-23a, miR-23b, miR-122-5p, miR-143, mir-145, miR-146a, miR-199a, miR-200b, miR-217, let-7f, let-7i, miR-130b, miR-29a, miR-132, miR-21, miR-375, miR-24, miR-31a, miR-192, miR-221, and miR-223. We conducted primer test and sample test after the total RNA was prepared. After that, we performed reverse transcription. We prepared the PCR reaction plate after creating and establishing a plate document, ran the PCR reaction plate, and then analyzed the results. The main steps were as follows: High throughput sequencing was performed on individual samples. Using the one-step method, total RNA was extracted from by TRIzol (Invitrogen, Gaithersburg, MD, USA). RNA concentrations were analyzed using Nanodrop (Nanodrop Technologies, Wilmington, DE, USA) after purification, and quality testing was conducted using BioAnalyzer (Agilent Technologies, Palo Alto, CA, USA). The small RNA was purified from the total RNA, and the molecules within the range of 16–30 nt were enriched. Then, the 3' and 5' sequences were attached, and cDNA was synthesized by Superscript II reverse transcriptase. Finally, PCR amplification was performed.
The behavior and stools of the rats showed no change after completing the models. When the model rats were frightened or underwent intragastric administration, they experienced an excessive reaction.
The rectum affusion amount of the rats in the model group (0.77±0.08 mL) was lower than that in the control group (1.52±0.09 mL), and the difference was significant (p<0.01). Thus, the sensitivity of model rats was higher, suggesting that model building method was successful.
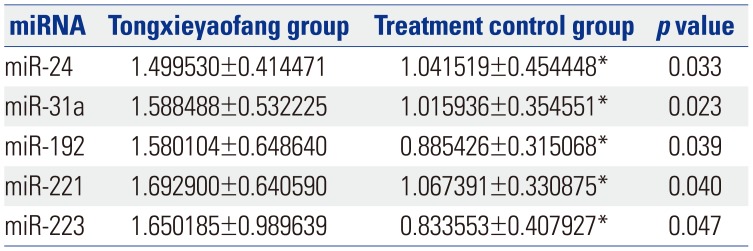
Fig. 1 shows a heat map of the expression of the 21 miRNAs. The expression of miR-23a, miR-23b, miR-122-5p, miR-143, mir-145, miR-146a, miR-199a, miR-200b, and miR-217 showed no differences between control group and model group (Table 1). The expression of let-7f, let-7i, miR-130b, miR-29a, miR-132, miR-21, and miR-375 was up-regulated (p<0.05) (Table 2), while the expression of miR-24, miR-31a, miR-192, miR-221, and miR-223 was down-regulated (p<0.05) (Table 3) in the colons of IBS rats. After treatment with Tongxieyaofang, the expression of let-7f, let-7i, miR-130b, miR-29a, miR-132, miR-21, and miR-375 was lower, compared with the treatment control group (p<0.05) (Table 4), while the expression of miR-24, miR-31a, miR-192, miR-221, and miR-223 was higher (p<0.05) (Table 5).
IBS is recognized as one of the most common disorders among functional gastrointestinal disorders, and the main symptoms of this functional disease are abdominal pain and stool irregularities.23 IBS appears to affect up to 20% of the population in the Western world at any given time.24 The median value of IBS prevalence in Asian countries ranges 6.5–10.1%.25 The pathogenesis of IBS is mainly focused on the following aspects: motility disorders, altered visceral sensitivity and pain processing, increased mucosal permeability, altered microbiota, and low grade inflammation.26 Visceral hypersensitivity has been an important role in the pathogenesis of IBS. Therapy for IBS is primarily targeted at treating the symptoms experientially1; nonetheless, symptom treatment has shown limited success and cannot be effective for long-term management.
Another factor likely involved in the mechanisms of IBS is miRNA. MiRNAs were identified as small, endogenous, non-coding RNAs that have the capacity for gene regulation after transcription.27 Several studies have indicated that miRNAs can be used as a marker in the diagnosis of gastrointestinal diseases, and in patients who have IBS, miRNAs expression is altered in colonic tissue. These miRNAs regulate intestinal pathways that result in epigenetic and genetic events.5 In our study, we found that the expression of let-7f, let-7i, miR-130b, miR-29a, miR-132, miR-21, and miR-375 was higher in visceral hypersensitivity model rats. A previous study showed the let-7 family played a key role in the progression of atherosclerosis and intracranial aneurysm.28 Also, miRNA let-7i was found to regulate dendritic cell maturation targeting interleukin (IL)-10 via the Janus kinase 1-signal transducer.29 Another study30 reported let-7f-miRNA negatively regulates hepatic differentiation of human adipose tissue-derived stem cells. Moreover, one study31 reported that an increased number of dendritic cells in the colon stimulated CD4(+) T cells to secrete high levels of IL-4, which led to the activation of dendritic cells and subsequently resulted in visceral hypersensitivity. With the high expression of let-7f and let-7i, we speculate that the mechanism of IBS is connected with dendritic cells and regulation of let-7 gene family. Rieger, et al.32 reported that the increased miR-130b expression is a potential negative regulator of drug metabolism by directly and/or indirectly affecting the expression of several ADME genes that may be relative to pathophysiologic conditions, such as cholestasis and inflammation, which are associated with increased miR-130b expression. Therefore, we deduced IBS involving infection may be regulated directly and/or indirectly via miR-130b. It has been reported that miR-29a regulates both glutamine synthetase and intestinal membrane permeability in IBS patients.9 It was reported that a change in the balance of miR-132 and Ctbp2 interaction, as part of the larger developmental program, is involved in the transition from embryonic to larval spinal cord by gradually driving the switch in Notch output and radial glial progenitor fate.33 As one of the mechanisms in IBS is brain-gut axis, we suspect miR-132 plays an important role in the spinal cord of IBS rats. MiR-21 was found to be involved in the regulation of intestinal epithelial barrier function.34 MiR-375 was reported to play an important role in colon cancer,35 although the correlation between miR-375 and IBS should be studied in the future. On the contrary, we found the expression of miR-24, miR-31a, miR-192, miR-221, and miR-223 was lower in IBS rats. MiR-24 is reported to play a role in the pathogenesis of IBS, probably through regulating SERT expression.10 There is no study about miR-31a, miR-192, miR-221, and miR-223 in IBS.
Tongxieyaofang is a traditional and classical Chinese medical prescription in the treatment of diarrhea. A systematic review reported evidence indicating the potential usefulness thereof for IBS patients.36 Studies demonstrated that Tongxieyaofang had a significant analgesic effect on IBS3738 In our study, after treatment with Tongxieyaofang, the expression of let-7f, let-7i, miR-130b, miR-29a, miR-132, miR-21, and miR-375 was lower than that in the control group treated with saline, while the expression of miR-24, miR-31a, miR-192, miR-221, and miR-223 was higher. These results indicated that miRNAs are involved in the mechanism of visceral hypersensitivity and are connected with treatment, such that they might be targets for treating IBS. However, further studies are needed to explain the specific mechanism of the treatment of Tongxieyaofang in IBS.
In conclusion, miRNAs play a pivotal role in the mechanism of visceral hypersensitivity and might be targets for treating IBS. Tongxieyaofang elicited differential expression of multiple miRNAs in visceral hypersensitivity rats. Further studies will need to be done to detect the interacting mechanism of miRNAs in IBS patients and to build expression profiles of miRNAs of use in diagnosis and treatment of IBS.
ACKNOWLEDGEMENTS
This research was supported by funding from Zhejiang Provincial Natural Science Foundation of China under Grant No. LY18H030001; the Medicine and Health Science and Technology Plan Projects in Zhejiang province (2017KY413), Traditional Chinese Medicine Science and Technology Plan of Zhejiang Province (2017ZA089, 2016ZB071, 2015ZZ012, 2014ZA030); National Natural Science Foundation of China (81573760); Medical Health Platform Plan Projects of Zhejiang Province (2015RCA020); Zhejiang Provincial Natural Science Foundation of China (LY16H030010).
References
1. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006; 130:1480–1491. PMID: 16678561.

2. Verne GN, Robinson ME, Price DD. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain. 2001; 93:7–14. PMID: 11406333.

3. Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009; 146:41–46. PMID: 19595511.

4. Camilleri M, Nadeau A, Lamsam J, Nord SL, Ryks M, Burton D, et al. Understanding measurements of intestinal permeability in healthy humans with urine lactulose and mannitol excretion. Neurogastroenterol Motil. 2010; 22:e15–e26. PMID: 19614866.

5. Zhou Q, Verne GN. New insights into visceral hypersensitivity--clinical implications in IBS. Nat Rev Gastroenterol Hepatol. 2011; 8:349–355. PMID: 21643039.

6. Zhou Q, Yang L, Larson S, Basra S, Merwat S, Tan A, et al. Decreased miR-199 augments visceral pain in patients with IBS through translational upregulation of TRPV1. Gut. 2016; 65:797–805. PMID: 25681400.

7. Kim J, Krichevsky A, Grad Y, Hayes GD, Kosik KS, Church GM, et al. Identification of many microRNAs that copurify with polyribosomes in mammalian neurons. Proc Natl Acad Sci U S A. 2004; 101:360–365. PMID: 14691248.

8. Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010; 9:775–789. PMID: 20885409.

9. Zhou Q, Souba WW, Croce CM, Verne GN. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut. 2010; 59:775–784. PMID: 19951903.

10. Liao XJ, Mao WM, Wang Q, Yang GG, Wu WJ, Shao SX. MicroRNA-24 inhibits serotonin reuptake transporter expression and aggravates irritable bowel syndrome. Biochem Biophys Res Commun. 2016; 469:288–293. PMID: 26631964.

11. Fourie NH, Peace RM, Abey SK, Sherwin LB, Rahim-Williams B, Smyser PA, et al. Elevated circulating miR-150 and miR-342-3p in patients with irritable bowel syndrome. Exp Mol Pathol. 2014; 96:422–425. PMID: 24768587.

12. Yang QQ, Xu XP, Zhao HS, Cai YQ, Pan YM, Xu JQ, et al. Differential expression of microRNA related to irritable bowel syndrome in a rabbit model. J Dig Dis. 2017; 18:330–342. PMID: 28509372.

13. Johnston DGW, Williams MA, Thaiss CA, Cabrera-Rubio R, Raverdeau M, McEntee C, et al. Loss of microRNA-21 influences the gut microbiota, causing reduced susceptibility in a murine model of colitis. J Crohns Colitis. 2018; 12:835–848. PMID: 29608690.

14. Ma G, Dai W, Sang A, Yang X, Gao C. Upregulation of microRNA-23a/b promotes tumor progression and confers poor prognosis in patients with gastric cancer. Int J Clin Exp Pathol. 2014; 7:8833–8840. PMID: 25674252.
15. Xu X, Gao F, Wang J, Tao L, Ye J, Ding L, et al. MiR-122-5p inhibits cell migration and invasion in gastric cancer by down-regulating DUSP4. Cancer Biol Ther. 2018; 19:427–435. PMID: 29509059.

16. Lin XT, Zheng XB, Fan DJ, Yao QQ, Hu JC, Lian L, et al. MicroRNA-143 targets ATG2B to inhibit autophagy and increase inflammatory responses in Crohn's disease. Inflamm Bowel Dis. 2018; 24:781–791. PMID: 29562274.

17. Kim H, Banerjee N, Sirven MA, Minamoto Y, Markel ME, Suchodolski JS, et al. Pomegranate polyphenolics reduce inflammation and ulceration in intestinal colitis-involvement of the miR-145/p70S6K1/HIF1α axis in vivo and in vitro. J Nutr Biochem. 2017; 43:107–115. PMID: 28282584.

18. Szuücs D, Béres NJ, Rokonay R, Boros K, Borka K, Kiss Z, et al. Increased duodenal expression of miR-146a and -155 in pediatric Crohn's disease. World J Gastroenterol. 2016; 22:6027–6035. PMID: 27468194.
19. Shen Y, Zhou M, Yan J, Gong Z, Xiao Y, Zhang C, et al. miR-200b inhibits TNF-α-induced IL-8 secretion and tight junction disruption of intestinal epithelial cells in vitro. Am J Physiol Gastrointest Liver Physiol. 2017; 312:G123–G132. PMID: 27979826.

20. Heckmann D, Maier P, Laufs S, Li L, Sleeman JP, Trunk MJ, et al. The disparate twins: a comparative study of CXCR4 and CXCR7 in SDF-1α-induced gene expression, invasion and chemosensitivity of colon cancer. Clin Cancer Res. 2014; 20:604–616. PMID: 24255072.

21. Peck BC, Mah AT, Pitman WA, Ding S, Lund PK, Sethupathy P. Functional transcriptomics in diverse intestinal epithelial cell types reveals robust MicroRNA sensitivity in intestinal stem cells to microbial status. J Biol Chem. 2017; 292:2586–2600. PMID: 28053090.

22. Olaru AV, Selaru FM, Mori Y, Vazquez C, David S, Paun B, et al. Dynamic changes in the expression of MicroRNA-31 during inflammatory bowel disease-associated neoplastic transformation. Inflamm Bowel Dis. 2011; 17:221–231. PMID: 20848542.

23. Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006; 130:1377–1390. PMID: 16678553.

24. Thompson WG, Heaton KW, Smyth GT, Smyth C. Irritable bowel syndrome in general practice: prevalence, characteristics, and referral. Gut. 2000; 46:78–82. PMID: 10601059.

25. Chang FY, Lu CL, Chen TS. The current prevalence of irritable bowel syndrome in Asia. J Neurogastroenterol Motil. 2010; 16:389–400. PMID: 21103420.

26. Corsetti M, Van Oudenhove L, Tack J. The quest for biomarkers in IBS-where should it lead us? Neurogastroenterol Motil. 2014; 26:1669–1676. PMID: 25424580.

27. Farh KK, Grimson A, Jan C, Lewis BP, Johnston WK, Lim LP, et al. The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science. 2005; 310:1817–1821. PMID: 16308420.

28. Sima X, Sun H, Zhou P, You C. A potential polymorphism in the promoter of let-7 is associated with an increased risk of intracranial aneurysm: a case-control study. Medicine (Baltimore). 2015; 94:e2267. PMID: 26705209.
29. Sun Y, Jin X, Liu X, Zhang M, Liu W, Li Z, et al. MicroRNA let-7i regulates dendritic cells maturation targeting interleukin-10 via the Janus kinase 1-signal transducer and activator of transcription 3 signal pathway subsequently induces prolonged cardiac allograft survival in rats. J Heart Lung Transplant. 2016; 35:378–388. PMID: 26755202.

30. Davoodian N, Lotfi AS, Soleimani M, Mola SJ, Arjmand S. Let-7f microRNA negatively regulates hepatic differentiation of human adipose tissue-derived stem cells. J Physiol Biochem. 2014; 70:781–789. PMID: 25077652.

31. Li M, Zhang L, Lu B, Chen Z, Chu L, Meng L, et al. Role of dendritic cell-mediated abnormal immune response in visceral hypersensitivity. Int J Clin Exp Med. 2015; 8:13243–13250. PMID: 26550249.
32. Rieger JK, Reutter S, Hofmann U, Schwab M, Zanger UM. Inflammation-associated microRNA-130b down-regulates cytochrome P450 activities and directly targets CYP2C9. Drug Metab Dispos. 2015; 43:884–888. PMID: 25802328.

33. Salta E, Lau P, Sala Frigerio C, Coolen M, Bally-Cuif L, De Strooper B. A self-organizing miR-132/Ctbp2 circuit regulates bimodal notch signals and glial progenitor fate choice during spinal cord maturation. Dev Cell. 2014; 30:423–436. PMID: 25132384.

34. Chen WX, Ren LH, Shi RH. Implication of miRNAs for inflammatory bowel disease treatment: systematic review. World J Gastrointest Pathophysiol. 2014; 5:63–70. PMID: 24891977.

35. Mussnich P, Rosa R, Bianco R, Fusco A, D'Angelo D. MiR-199a-5p and miR-375 affect colon cancer cell sensitivity to cetuximab by targeting PHLPP1. Expert Opin Ther Targets. 2015; 19:1017–1026. PMID: 26107137.

36. Bian Z, Wu T, Liu L, Miao J, Wong H, Song L, et al. Effectiveness of the Chinese herbal formula TongXieYaoFang for irritable bowel syndrome: a systematic review. J Altern Complement Med. 2006; 12:401–407. PMID: 16722791.

37. Liao H, Banbury LK, Leach DN. Elucidation of danzhixiaoyao wan and its constituent herbs on antioxidant activity and inhibition of nitric oxide production. Evid Based Complement Alternat Med. 2007; 4:425–430. PMID: 18227909.

38. Hu XG, Xu D, Zhao Y, Yang XB, Meng J, Shen H, et al. The alleviating pain effect of aqueous extract from tong-xie-yao-fang, on experimental visceral hypersensitivity and its mechanism. Biol Pharm Bull. 2009; 32:1075–1079. PMID: 19483318.

Fig. 1
Expression of miRNAs in the rat colon. Group 1, control group; Group 2, model control group; Group 3, treatment control group; Group 4, Tongxieyaofang group.

Table 1
miRNA Showed No Differences in Visceral Hypersensitivity Rats in the Model Group
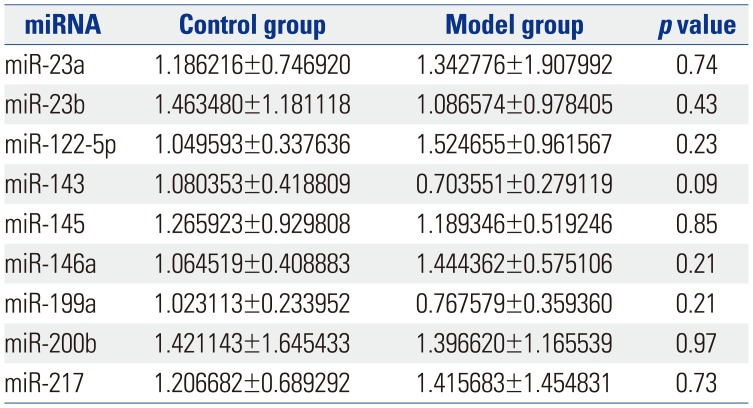
Table 2
Up-Regulated miRNA in the Colons of Visceral Hypersensitivity Rats in the Model Group
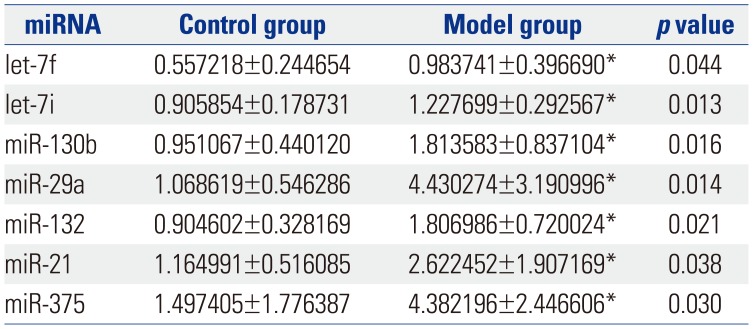
Table 3
Down-Regulated miRNA in the Colons of Visceral Hypersensitivity Rats in the Model Group
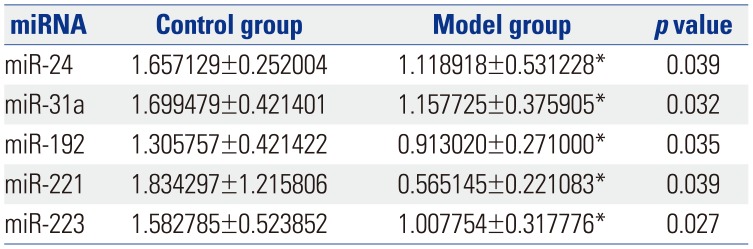
Table 4
Down-Regulated miRNA in Visceral Hypersensitivity Rats after Tongxieyaofang Treatment
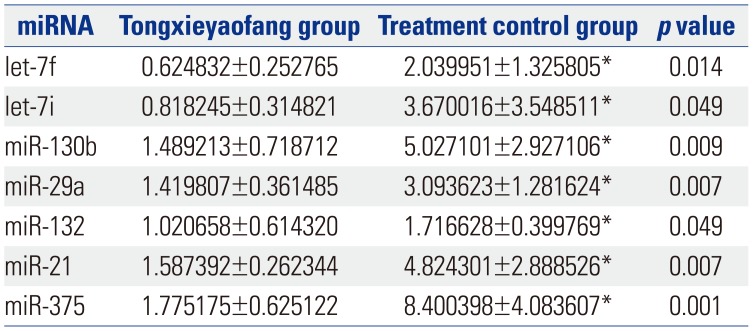
Table 5
Up-Regulated miRNA in Visceral Hypersensitivity Rats after Treatment with Tongxieyaofang




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download