Abstract
Objectives
To investigate if vitamin D receptor (VDR) gene polymorphisms and circulating vitamin D levels are associated with pelvic floor disorders (PFDs).
Methods
In this case-control study, 25-hydroxy-vitamin D (25[OH]D) serum levels were analyzed in 47 females with PFDs and 87 healthy females (controls), respectively. The VDR gene polymorphisms were determined by using polymerase chain reaction and performing digestions with 4 restriction enzymes i.e., ApaI, TaqI, FokI, and BsmI. Vitamin D levels of patients were divided into <20 ng/mL, 20 to 30 ng/mL, and ≥30 ng/mL categories.
Results
Our correlative analysis of VDR polymorphisms as a function of the presence of PFD showed that ApaI and BsmI polymorphisms were significantly associated with PFD in vitamin-D-deficiency and insufficiency groups (P < 0.05). Mean vitamin D levels did not differ between the PFD case (13.01 ± 0.84 ng/mL) and control (15.11 ± 1.04 ng/mL) groups (P > 0.05). However, there was a significant difference in the distribution of vitamin D levels between study group and controls using Pearson's χ2 test (<20 ng/mL, 20–30 ng/mL, and >30 ng/mL: 87.2%, 12.8%, and 0% in the study group and 75.9%, 16.1%, and 8.0% in controls, respectively, P < 0.05). Taken together, our observations suggest that vitamin D levels could be associated with PFDs and that 2 polymorphisms (i.e., ApaI and BsmI) in the VDR gene may contribute to an increased prevalence of PFDs in women with insufficient levels of vitamin D.
Pelvic floor disorders (PFDs) are major health problems that can significantly compromise quality of life for affected women. In especially postmenopausal women, PFDs are important issues with heart disease, osteoporosis, and Alzheimer's disease.12 Almost 24% of women are reported to be living with at least one PFD such as urinary incontinence (UI), fecal incontinence (FI) and pelvic organ prolapse (POP).345 The female pelvic floor is composed of the levator ani and coccygeus skeletal muscles. These muscles function together to support the visceral contents of the abdominal cavity through sophisticated relationships between ligamentous connective tissue and skeletal muscles. Skeletal and smooth muscles are involved in the function and support of all pelvic viscera.6 Pelvic floor muscle weakness is clinically observed in women that display PFD symptoms7 through vaginal bulge or protrusion,8 involuntary leakage of urine, flatus or feces.3 Causes of PFD are most likely multifactorial. PFD has previously been associated with age,9 pregnancy,10 childbirth,11 hysterectomy,12 obesity,111314 race,14 functional impairment, and cognitive impairment.10
Vitamin D is a fat-soluble micronutrient that plays a vital role in calcium and phosphate homeostasis in smooth and skeletal muscle.1516 Vitamin D levels are determined by many factors, such as skin pigmentation, geographic location, and body mass index (BMI).61718 Several studies suggest that serum vitamin D levels may impact functional efficiency of skeletal muscle by regulating calcium homeostasis that in turn affects muscle contractility and by protecting the muscle cellular environment against insulin resistance and inflammation.1719202122 Vitamin D deficiency (described as exhibiting a c] serum level <20 ng/mL) has long been clinically associated with impaired muscle strength and loss of muscle mass.23 Vitamin D insufficiency (25-hydroxy-vitamin D (25[OH]D) serum level 20–30 ng/mL) is a milder form of vitamin D deficiency, affecting 38% to 73% of different populations,6 and has been related with muscle strength and mobility.20
Vitamin D can mediate its effects on muscle through the formation of a complex with a vitamin D receptor (VDR).1624 The VDR gene contains 4 polymorphic regions, each named after the restriction enzymes used to detect them: ApaI (rs7975232), TaqI (rs731236), FokI (rs2228570) and BsmI (rs1544410).2526 Although many studies have demonstrated that VDR gene polymorphisms are associated with muscle strength272829 and bone mineral density (BMD),27 little research has examined the association of PFD with vitamin D status in Korean women. We conducted a case-control study to evaluate the impact of vitamin D status on PFD symptoms in Korean women, and performed an association analysis between the ApaI, TaqI, FokI, and BsmI VDR polymorphisms and PFD in the same population.
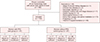
The data set comprised 180 women who utilized health examination center and the Obstetrics and Gynecology Clinic at Seoul Medical Center between June and November 2014. All participants provided written informed consent prior to study commencement. This study was approved by the Institutional Review Board of Seoul Medical Center (approval no. 2014-017). Women were excluded if they had any medical conditions that impair vitamin D absorption or metabolism or are known to be a major cause of PFD, including: stage ≥3 chronic kidney disease, chronic liver disease, neurologic disease (cerebral vascular accident, or spinal cord injury), complications of diabetes mellitus with end stage disease (retinopathy, neuropathy, ophthalmic complications, or amputations), gastric bypass, rectovaginal fistula, pelvic floor surgery or pelvic irradiation. Pregnant women were excluded. Patients known to be on vitamin D supplementation were excluded in order to ensure a more homogeneous cohort. Ultimately, 134 women were included in this study (Fig. 1). All eligible participants had a blood sample taken and had sufficient serum available for vitamin D assays. Serum vitamin D panels were processed using liquid chromatography.15 The 25(OH)D panel included both total 25(OH) D as well as 25(OH)D2 and D3 fractions.
First, a number of standard characteristics were collected for all 134 patients. Using a standard data form, the Electronic Medical Record was abstracted to obtain demographic, medical, and laboratory data. Demographic (i.e., age, height, weight, parity, and status of menopause) and medical characteristics were obtained from the initial history and physical examination documentation. BMI was calculated and reported as kilograms/square meter. Parity (total number of vaginal and cesarean deliveries) was used as a continuous variable in some analyses and categorized as none, 1, 2, 3, or 4 or more births in others.
To identify women with PFD symptoms and divide the data set into cases and controls, all participants filled in standardized questionnaires including a Urinary Distress Inventory (UDI-6), Colorectal Anal Distress Inventory (CRADI-8), POP Distress Inventory (POPDI-6), and Incontinence Impact Questionnaire (IIQ-7). Definitions for UI, FI, and POP were identical to those used by the PFDs Network in their article describing the prevalence of these disorders in women surveyed in the 2005 to 2006 the National Health and Nutrition Examination Survey (NHANES).23 Specifically, classification of UI was based on the responses to frequency and the amount of leakage (2 NHANES questions) summarized as the “incontinence severity index”. Women with a score of 3 or higher were considered to be incontinent.3 FI was defined as at least monthly leakage of solid, liquid, or mucous stool, also based on responses to a combination of type and frequency of symptom question.3 POP was considered positive if individuals answered yes to the question, “Do you experience bulging or something falling out you can see or feel in the vaginal area?”, which was derived from the Pelvic Floor Distress Inventory.3 Based on these questionnaires, the 134 women in the data set were divided into a case group that had PFD (n = 47) and a control group (n = 87) that did not have PFD. The case and control group were further trichotomized based on vitamin D status as deficient, insufficient and sufficient. For the purposes of this evaluation, vitamin D deficiency was defined as a total serum vitamin D level <20 ng/mL.15 Women with total serum vitamin D levels ≥30 ng/mL were classified as being vitamin D sufficient; women with serum levels 20 to 30 ng/mL were grouped as vitamin D insufficient.
Genomic DNA was extracted from ethylenediamine-tetraceticacid (EDTA) whole blood using a salting-out procedure. Genotyping of the following VDR gene polymorphisms was performed using a QIAamp DNA Blood Mini Kit (Qiagen, Inc., Valencia, CA, USA): ApaI (rs7975232), TaqI (rs731236), FokI (rs2228570) and BsmI (rs1544410). These polymorphisms were named after the restriction enzyme that have traditionally been used for their detection.24 The VDR gene polymorphisms were analyzed using polymerase chain reaction-restriction fragment length polymorphism analysis.
Data were analyzed with significance determined at P < 0.05 using SPSS version 24.0 (SPSS Inc., Chicago, IL, USA) and values in the text are expressed as means ± standard error of mean. Student's t-tests or one-way analysis of variance (ANOVA) were used for comparisons between PFD and non-PFD group (age, BMI, parity, menopause status and serum total vitamin D levels). Pearson's χ2 test was also used for VDR genotype distribution analysis in PFD.
One hundred and eighty women who had a serum vitamin D level drawn between June and November, 2014, attended health examination center and the Obstetrics and Gynecology Clinic at Seoul Medical Center were considered in this analysis. Forty-six women were excluded due to the presence of stage III chronic kidney disease, chronic liver disease, neurologic disease, diabetes mellitus with end stage disease, pelvic floor surgery, pelvic irradiation or vitamin D therapy (Fig. 1). Of the remaining 134 women, 47 cases had PFD symptoms, and 87 controls had no PFD symptoms. Each group was further sub-categorized according to vitamin D levels (deficient <20 ng/mL, insufficient 20–30 ng/mL, and sufficient ≥30 ng/mL; Fig. 1).
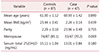
Our study aimed to identify whether vitamin D status was associated with PFD symptoms. The mean serum vitamin D level in the cases (13.01 ± 0.84) was not significantly different to that of the control group (15.11 ± 1.04) (Table 1). However, there were significant differences between the case and control groups within vitamin D insufficient/deficient women (P < 0.05; Table 2). In the case group, 87.2% (41/47) of the women were vitamin D deficient, 12.8% (6/47) were vitamin D insufficient, and 0.0% (0/47) were vitamin D sufficient. In the control group, 75.9% (66/87) were vitamin D deficient, 16.1% (14/87) were vitamin D insufficient, and 8.0% (7/87) were vitamin D sufficient (Table 2).
In the χ2 analysis between VDR polymorphisms and PFD, there were no significant differences in PFD diagnosis proportions between the cases and controls for the TaqI and FokI polymorphisms. However, the ApaI and BsmI polymorphisms were significantly associated with PFD in the vitamin D deficient and insufficient groups (Table 3). The cases showed an increased frequency of Aa genotype in the ApaI polymorphism compared to the frequency of the Aa genotype in this polymorphism in the controls (P < 0.05). The frequencies of AA and aa genotypes in ApaI were not increased in the cases compared to the controls in the vitamin D deficient and insufficient groups (P > 0.05). In addition, the frequency of bb genotype in Bsm1 was significantly higher in the cases, while the frequency of Bb genotype in BsmI was significantly higher in the controls (P < 0.05). Using χ2, ApaI and BsmI VDR polymorphisms are linked, as significant differences in the genotypic frequencies between the cases and controls were observed for Aa × bb genotype (22.5% vs. 46.8%, respectively, P < 0.05; Table 4).
This study demonstrated that serum vitamin D status correlates inversely with the prevalence of PFD in women. All women with PFD symptoms in this study were vitamin D insufficient or deficient, defined as exhibiting a 25(OH)D serum level <30 ng/mL or <20 ng/mL. The vitamin D deficient group had a higher PFD incidence rate than the insufficient group. Furthermore, we demonstrated that there was a significant difference in the distribution of VDR genotypes between the cases and controls in vitamin D sufficient/deficient women. To analyze the association between the VDR genotypes and prevalence of PFD, we used the restriction enzymes ApaI, TaqI, FokI and BsmI to genotype 4 polymorphisms in the individuals, each polymorphism named after the restriction enzyme used to genotype it: ApaI (rs7975232), TaqI (rs731236), FokI (rs2228570), and BsmI (rs1544410). The Aa (ApaI) and bb (BsmI) genotype frequencies were higher in the cases than in the controls. To our knowledge, this is the first study to demonstrate an association between VDR gene polymorphisms and PFD.
Our findings are supported by previous clinical studies suggesting that vitamin D deficiency is more prevalent in women with PFD than in the general population. Parker-Autry and colleagues7 performed a retrospective study (n = 394) and found that women with PFD had significantly lower levels of vitamin D (29.3 ± 11.5 ng/mL) cmopared to women without PFD (35.0 ± 14.1 ng/mL) (P < 0.001). In addition, this study demonstrated that higher CRADI-8 scores and IIQ-7 scores were associated with vitamin D insufficiency.7 Badalian and Rosenbaum23 performed a cross-sectional analysis using 2005 to 2006 the NHNES data (n = 1,881) and observed that mean vitamin D levels were significantly lower for women with PFD. In addition, they showed significantly decreased risks of PFD with increasing vitamin D levels in all women aged 20 or older (odds ratio [OR] = 0.94; 95% confidence interval [CI], 0.88–0.99) and in subset of women 50 years and older (OR = 0.92; 95% CI, 0.85–0.99).23
Vitamin D increases skeletal muscle cell proliferation and muscle fiber size in vitro.3031 Vitamin D may be instrumental for skeletal muscle functional efficiency by regulating calcium homeostasis to affect muscle contractility and by protecting the muscle cellular environment against inflammation.6 Thus, insufficient vitamin D levels may affect various pathophysiologic pathways and contribute to PFDs by disrupting VDR expression and calcium homeostasis in pelvic floor skeletal and visceral musculature.6 These mechanisms may explain our observation of increased vitamin D deficiency in women with PFD symptoms compared to controls.
Previous studies show that VDR gene polymorphisms are associated with muscle strength, BMD, and various diseases. 2728293233 As far as we know, no previous studies have investigated the effect of the VDR gene polymorphisms on PFD. In this study, we demonstrated that VDR gene polymorphisms were not associated with serum 25(OH)D levels. Although no overall association was found between TaqI or FokI and the prevalence of PFD, we observed a significant association between ApaI or BsmI genotypes and PFD in vitamin D insufficient/deficient women. Regarding the ApaI polymorphism, the frequency of heterozygotes (Aa) was significantly higher in the cases than in the controls, and the frequency of homozygotes (AA and aa) was lower in cases than in controls in vitamin D insufficient/deficient women. Although ApaI-composition of common population3435 are more heterozygotes than homozygotes, we showed that frequency of aa genotype in non-PFD group was highest. Therefore, it is plausible that the prevalence of PFD in heterozygote women may be higher than in homozygote women.
BsmI is also associated with skeletal muscle function. The b (rather than B) allele is associated with lower fat-free mass and hamstring strength in healthy women aged 20 to 39 years, whereas, in non-obese elderly women, both handgrip and quadriceps strength are higher in those with the bb genotype.2728 Barr et al.36 reported that the bb homozygote of Bsm1 is associated with higher serum 25(OH) D, lower parathyroid hormone and stronger measures of muscle force. Our report showed that the frequency of bb homozygotes in the cases was significantly higher than in the controls, and the heterozygote frequency was lower in cases than in the controls in vitamin D insufficient/deficient women. Furthermore, the frequency of a combination of ApaI and BsmI genotypes (Aa/bb) was significantly higher in the PFD group in vitamin D insufficient/deficient women and was double the number of individuals with the same genotype in the non-PFD group. Both the ApaI and BsmI polymorphisms are located at the 3′ end of the VDR gene.24 It is reported that the ApaI, BsmI, and TaqI polymorphisms are in strong linkage disequilibrium. Morrison et al.37 reported that the BsmI and ApaI restriction sites are in close proximity in Caucasian populations. In young Chinese women research, Wang et al.33 showed that VDR gene ApaI and BsmI polymorphisms rather than TaqI were associated with muscular strength. Therefore, we suggest that vitamin D insufficient/deficient women with an Aa/bb genotype may have a higher risk of developing PFD.
Several limitations of this study should be addressed. First, sample sizes are limited; thus only large genotype effects could be detected and there was not enough statistical power to detect genotype interaction effects. The size of the vitamin D sufficient group was too small to compare with vitamin D insufficient/deficient women. Second, the race of the subjects was limited to Koreans. VDR genotypes vary among racial groups; therefore, a variety of studies in different races will be needed to obtain a fuller understanding.
In conclusion, vitamin D insufficiency may influence the prevalence of PFD in ways that are as yet unclear, possibly involving pelvic floor skeletal muscle weakness. Furthermore, the prevalence of PFD may be affected by variations in the VDR genotype. Our study provides meaningful results to understand the relationship between VDR gene polymorphisms and PFD. Particularly, the ApaI and BsmI genotypes of the VDR gene may be related to an increased prevalence of PFD in women. As vitamin D insufficiency of women is very common among Koreans (>90%),3839 we suggest that vitamin D status and VDR genotype analysis would be helpful for assessing the risk of PFDs in these women.
Figures and Tables
Acknowledgement
This study was supported by the research fund from Research Institute of Seoul Medical Center, Republic of Korea (grant#14-c06).
References
1. Yi SS, Hwang E, Baek HK, Kim TH, Lee HH, Jun HS, et al. Application of bioactive natural materials-based products on five women's diseases. J Menopausal Med. 2015; 21:121–125.

2. Song HJ, Kim TH, Lee HH, Kim JM, Park YJ, Lee A, et al. Cell therapy products in Alzheimer disease. J Menopausal Med. 2017; 23:1–4.

3. Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J, et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008; 300:1311–1316.

4. Greer WJ, Richter HE, Bartolucci AA, Burgio KL. Obesity and pelvic floor disorders: a systematic review. Obstet Gynecol. 2008; 112:341–349.
6. Parker-Autry CY, Burgio KL, Richter HE. Vitamin D status: a review with implications for the pelvic floor. Int Urogynecol J. 2012; 23:1517–1526.

7. Parker-Autry CY, Markland AD, Ballard AC, Downs-Gunn D, Richter HE. Vitamin D status in women with pelvic floor disorder symptoms. Int Urogynecol J. 2012; 23:1699–1705.

8. Roozbeh N, Darvish L. Acacia nilotica: new plant for help in pelvic organ prolapse. J Menopausal Med. 2016; 22:129–130.

9. Kepenekci I, Keskinkilic B, Akinsu F, Cakir P, Elhan AH, Erkek AB, et al. Prevalence of pelvic floor disorders in the female population and the impact of age, mode of delivery, and parity. Dis Colon Rectum. 2011; 54:85–94.

10. Hunskaar S, Arnold EP, Burgio K, Diokno AC, Herzog AR, Mallett VT. Epidemiology and natural history of urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2000; 11:301–319.

11. Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women's Health Initiative: gravity and gravidity. Am J Obstet Gynecol. 2002; 186:1160–1166.

12. Gürel H, Gürel SA. Pelvic relaxation and associated risk factors: the results of logistic regression analysis. Acta Obstet Gynecol Scand. 1999; 78:290–293.

13. Uustal Fornell E, Wingren G, Kjølhede P. Factors associated with pelvic floor dysfunction with emphasis on urinary and fecal incontinence and genital prolapse: an epidemiological study. Acta Obstet Gynecol Scand. 2004; 83:383–389.

14. Moalli PA, Jones Ivy S, Meyn LA, Zyczynski HM. Risk factors associated with pelvic floor disorders in women undergoing surgical repair. Obstet Gynecol. 2003; 101:869–874.

15. Parker-Autry CY, Gleason JL, Griffin RL, Markland AD, Richter HE. Vitamin D deficiency is associated with increased fecal incontinence symptoms. Int Urogynecol J. 2014; 25:1483–1489.

16. Kim TH, Lee HH, Kim JM, Choi SD, Lee A, Hwang SY, et al. Expression of vitamin D receptor in seminal vesicles of cholesterol formula mice. J Menopausal Med. 2015; 21:89–92.

17. Lips P, Binkley N, Pfeifer M, Recker R, Samanta S, Cohn DA, et al. Once-weekly dose of 8400 IU vitamin D(3) compared with placebo: effects on neuromuscular function and tolerability in older adults with vitamin D insufficiency. Am J Clin Nutr. 2010; 91:985–991.

18. Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D-lightful story. J Bone Miner Res. 2007; 22:Suppl 2. V28–V33.

19. Ward KA, Das G, Roberts SA, Berry JL, Adams JE, Rawer R, et al. A randomized, controlled trial of vitamin D supplementation upon musculoskeletal health in postmenarchal females. J Clin Endocrinol Metab. 2010; 95:4643–4651.

20. Zhu K, Austin N, Devine A, Bruce D, Prince RL. A randomized controlled trial of the effects of vitamin D on muscle strength and mobility in older women with vitamin D insufficiency. J Am Geriatr Soc. 2010; 58:2063–2068.

21. Bischoff HA, Stähelin HB, Dick W, Akos R, Knecht M, Salis C, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003; 18:343–351.

22. Dhesi JK, Jackson SH, Bearne LM, Moniz C, Hurley MV, Swift CG, et al. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing. 2004; 33:589–595.

23. Badalian SS, Rosenbaum PF. Vitamin D and pelvic floor disorders in women: results from the National Health and Nutrition Examination Survey. Obstet Gynecol. 2010; 115:795–803.
24. Windelinckx A, De Mars G, Beunen G, Aerssens J, Delecluse C, Lefevre J, et al. Polymorphisms in the vitamin D receptor gene are associated with muscle strength in men and women. Osteoporos Int. 2007; 18:1235–1242.

25. Ranganathan P. Genetics of bone loss in rheumatoid arthritis--role of vitamin D receptor polymorphisms. Rheumatology (Oxford). 2009; 48:342–346.

26. Arai H, Miyamoto K, Taketani Y, Yamamoto H, Iemori Y, Morita K, et al. A vitamin D receptor gene polymorphism in the translation initiation codon: effect on protein activity and relation to bone mineral density in Japanese women. J Bone Miner Res. 1997; 12:915–921.

27. Geusens P, Vandevyver C, Vanhoof J, Cassiman JJ, Boonen S, Raus J. Quadriceps and grip strength are related to vitamin D receptor genotype in elderly nonobese women. J Bone Miner Res. 1997; 12:2082–2088.

28. Grundberg E, Brändström H, Ribom EL, Ljunggren O, Mallmin H, Kindmark A. Genetic variation in the human vitamin D receptor is associated with muscle strength, fat mass and body weight in Swedish women. Eur J Endocrinol. 2004; 150:323–328.

29. Roth SM, Zmuda JM, Cauley JA, Shea PR, Ferrell RE. Vitamin D receptor genotype is associated with fat-free mass and sarcopenia in elderly men. J Gerontol A Biol Sci Med Sci. 2004; 59:10–15.

30. Bischoff-Ferrari HA, Borchers M, Gudat F, Dürmüller U, Stähelin HB, Dick W. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res. 2004; 19:265–269.

31. Bischoff HA, Borchers M, Gudat F, Duermueller U, Theiler R, Stähelin HB, et al. In stiu detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J. 2001; 33:19–24.
32. Vandevyver C, Vanhoof J, Declerck K, Stinissen P, Vandervorst C, Michiels L, et al. Lack of association between estrogen receptor genotypes and bone mineral density, fracture history, or muscle strength in elderly women. J Bone Miner Res. 1999; 14:1576–1582.

33. Wang P, Ma LH, Wang HY, Zhang W, Tian Q, Cao DN, et al. Association between polymorphisms of vitamin D receptor gene ApaI, BsmI and TaqI and muscular strength in young Chinese women. Int J Sports Med. 2006; 27:182–186.

34. Curran JE, Vaughan T, Lea RA, Weinstein SR, Morrison NA, Griffiths LR. Association of A vitamin D receptor polymorphism with sporadic breast cancer development. Int J Cancer. 1999; 83:723–726.

35. Balsa JA, Iglesias B, Peromingo R, Conde S, Vazquez C, San-Millan JL, et al. Vitamin D receptor polymorphisms in secondary hyperparathyroidism after Scopinaro’s biliopancreatic diversion. Obes Surg. 2010; 20:1415–1421.

36. Barr R, Macdonald H, Stewart A, McGuigan F, Rogers A, Eastell R, et al. Association between vitamin D receptor gene polymorphisms, falls, balance and muscle power: results from two independent studies (APOSS and OPUS). Osteoporos Int. 2010; 21:457–466.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download