Abstract
Objectives
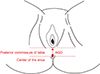
Anogenital distance (AGD) represents the space between labia posterior commissure and anus. This was pilot study to investigate how menopause and so lack of oestrogens affects AGD.
Methods
A total of 109 patients were enrolled. AGD was measured in lithotomy position using sterile paper ruler. Anogenital index (AGI) was used to control 2 variables of height and weight (body mass index, kg/m2). Vaginal health index (VHI) was used to evaluate vaginal wellness. Female sexual function index (FSFI) questionnaire was administered to all women to evaluate the impact of menopause on their sexual function.
Results
AGD (30.87 ± 2.98 vs. 17.57 ± 2.18; P = 0.0001) and AGI (1.40 ± 0.21 vs. 0.70 ± 0.15; P = 0.0001) were both significantly lower in the postmenopausal group. Postmenopausal women were affected by vulvovaginal atrophy (VVA) significantly. Thus, VHI scores were dramatically worse in postmenopausal group (23.95 ± 1.28 vs. 10.75 ± 3.41; P = 0.0001) as well as FSFI results (32.68 ± 2.25 vs. 19.78 ± 5.46; P = 0.0001).
The anogenital distance (AGD) is identified as the length between labia posterior commissure and the centre of the anus measured in millimetres. Its size depends on hormonal changes, particularly in estrogen and androgen level modifications.123 The relationship between AGD and sex steroids is still well known, considering that AGD has been used as bioindicator of fetal androgen exposure in humans and specifically to estimate the consequences of adverse in utero hormonal exposure.34567 Menopause hormonal variations are responsible of several anatomical and physiological changes in internal and external genitalia. Decreasing estrogen levels, the production of collagen changes with an increase in collagen strength, skin elasticity reduces and genitalia water content declines severely. Generally, genitalia trophism is dramatically affected by menopause, having in women a negative effect on intimacy and even on quality of life. Recent studies, confirm that one of the most severe menopause-related problem is vulvovaginal atrophy (VVA), involving more than 50% of women in Europe and reaching over 78% in Italian population.89
Nevertheless, there is a lack of studies on anatomical changes in adult females. This is a pilot study aimed to evaluate anatomical and morphological changes and comparison between pre-menopausal and post-menopausal women, focused on AGD and vaginal health index (VHI) modifications.
A total of 109 patients have been identified, recruited and enrolled in the outpatient clinic at the Department of Obstetrics and Gynecology, “Sapienza” University of Rome between April 2016 and December 2016.
Inclusion criteria for premenopausal group were: age between 20 and 45 years, regular periods in the past 1-year (intervals of 20–45 days). Inclusion criteria for premenopausal group were: age between 45 to 80 years, physiologic menopause since at least 1 year.
Exclusion criteria in both groups were: gynecological surgeries in the past affecting fertility and hormone production (e.g., oophorectomy), chemotherapy or irradiation of the pelvis, hormonal treatment in the previous year (combined oral contraceptives or hormone replacement therapy), congenital anatomical abnormalities, pregnancy, breastfeeding, severe comorbidities (e.g., autoimmune diseases, diabetes, etc.), infections, bleeding, and tumors.
All patients were extensively informed of study design and hypothesis. Written informed consent was obtained by all patients enrolled. Institutional Review Board (IRB) provided exempt to ethical approval. The present study was conducted following the principles of the Declaration of Helsinki. Candidates were consecutively randomized in the 2 groups.
Patients' data (height, weight, body mass index [BMI], AGD) were collected in the same day during outpatient examination. AGD measurement was conducted using a paper ruler in the lithotomy position to define the distance between posterior commissure of labia and anus centre (Fig. 1).
Anogenital index (AGI) was used to control 2 variables of height and weight,10 by dividing AGD by BMI (kg/m2).
VHI11 was used to evaluate vaginal wellness by analysing 5 parameters by clinical inspection: elasticity, fluid volume, pH, epithelial integrity and moisture (Table 1). Each parameter is graded from 1 (worst condition) to 5 (best condition). Scores ≤15 are considered to denote vaginal atrophy.
Female sexual function index (FSFI) questionnaire was administered to all women to evaluate the impact of genitalia changes on sexual life. FSFI is a validated, multidimensional, self-reported questionnaire to assess female sexual function.12 It consists of 19 items evaluating the FSFI lubrication, sexual arousal, orgasm, sexual satisfaction, and pain. The total score (range, 2–36; severe sexual dysfunction to full sexual function respectively) is calculated by summing the 5-domain score and interpreted by comparison with age- and population-dependent reference values for normal and impaired sexual function. A FSFI score ≤ 26.55 is considered to indicate sexual dysfunction.13
Interval data were analysed using the Mann-Whitney U test. Nominal data were evaluated using χ2 test or Fisher's exact test when appropriate. Parameters are expressed as as mean ± standard deviation (SD) and 95% confidence interval (CI). All statistical tests were performed using SPSS statistical software program (SPSS 20.0; SPSS Inc., Chicago, IL, USA). All P values of less than 0.05 were considered to indicate statistical significance.
To compare AGD in relation with VHI and FSFI of premenopausal and postmenopausal women, a total of 109 subjects were examined, being 48 in premenopause and 61 in menopause.
Patients' characteristics are shown in Table 2. BMI significantly varied between 2 groups (22.34 ± 2.84 vs. 25.36 ± 3.59; P = 0.0001), probably in relation to metabolic changes happening during menopause. AGD (30.87 ± 2.98 vs. 17.57 ± 2.18; P = 0.0001) and AGI (1.40 ± 0.21 vs. 0.70 ± 0.15; P = 0.0001) were both significantly lower in the postmenopausal group. Comparison between AGD in the 2 groups is evaluable in Figure 2 as well.
As highlighted in Table 2, postmenopausal women were affected by VVA significantly and negatively compared with premenopausal patients. Thus, VHI scores were dramatically worse in postmenopausal group (23.95 ± 1.28 vs. 10.75 ± 3.41; P = 0.0001) as well as FSFI results (32.68 ± 2.25 vs. 19.78 ± 5.46; P = 0.0001). Specific data on VHI and FSFI are shown in Figure 3 and 4 respectively.
AGD has been frequently investigated in newborn infants and adult males but lacking evidences are present in women.14 Moreover, in animal tests, AGD was usually used to evaluate drug toxicity. For instance, phthalates are synthetic chemical compound used in cosmetics and it have been demonstrated as responsible of a shortening of AGD in rodents.51516
In fact, AGD may be considered as an alternative biomarker of foetal testicular function, reflecting androgen action during the masculinisation process in animal models. This developmental androgen exposure allows normal differentiation and subsequent growth of male reproductive organs.32021
To our knowledge there is just another study investigating on AGD modifications before and after menopause.11 Lee and colleagues11 analysed 50 women (25 premenopause and 25 postmenopause) suggesting AGD and AGI as possible physical marker of menopausal aging of female genitalia.
As in our study, AGD was significantly longer in premenopausal women compared to postmenopausal women (34.8 ± 6.4 vs. 30.3 ± 6.6, P = 0.019). AGI was significantly higher in premenopausal women than postmenopausal women (1.7 ± 0.4 vs. 1.3 ± 0.3, P < 0.0001).
Menopause is responsible of several female physical changes having an important influence on the whole body. Skin elasticity, bone production/resorption, female genitalia trophism or function and cardiovascular system are entirely influenced by hormonal changes occurring in postmenopause.
This study confirms that AGD in postmenopausal women was significantly shorter than AGD in premenopausal women, correlating with an increase of VVA and sexual impairment.
Probably, AGD length modification can be the result of a decrease in collagen production, atrophoderma, a drop-in water content and epidermal thickness reduction caused by the decreased production of sex hormones after menopause.
Figures and Tables
 | Fig. 2Comparison between premenopause and postmenopause values of anogenital distance (AGD) (P < 0.0001). |
 | Fig. 3Distribution of vaginal health index (VHI) scores in the premenopausal and postmenopausal group. |
References
1. Mendiola J, Roca M, Mínguez-Alarcón L, Mira-Escolano MP, López-Espín JJ, Barrett ES, et al. Anogenital distance is related to ovarian follicular number in young Spanish women: a cross-sectional study. Environ Health. 2012; 11:90.

2. Basaran M, Kosif R, Bayar U, Civelek B. Characteristics of external genitalia in pre- and postmenopausal women. Climacteric. 2008; 11:416–421.

3. Dean A, Sharpe RM. Clinical review: anogenital distance or digit length ratio as measures of fetal androgen exposure: relationship to male reproductive development and its disorders. J Clin Endocrinol Metab. 2013; 98:2230–2238.
4. Barrett ES, Parlett LE, Redmon JB, Swan SH. Evidence for sexually dimorphic associations between maternal characteristics and anogenital distance, a marker of reproductive development. Am J Epidemiol. 2014; 179:57–66.

5. Swan SH, Sathyanarayana S, Barrett ES, Janssen S, Liu F, Nguyen RH, et al. First trimester phthalate exposure and anogenital distance in newborns. Hum Reprod. 2015; 30:963–972.

6. Courant F, Antignac JP, Maume D, Monteau F, Andersson AM, Skakkebaek N, et al. Exposure assessment of prepubertal children to steroid endocrine disrupters 1. Analytical strategy for estrogens measurement in plasma at ultratrace level. Anal Chim Acta. 2007; 586:105–114.
7. Barrett ES, Parlett LE, Sathyanarayana S, Liu F, Redmon JB, Wang C, et al. Prenatal exposure to stressful life events is associated with masculinized anogenital distance (AGD) in female infants. Physiol Behav. 2013; 114-115:14–20.

8. Nappi RE, Palacios S, Particco M, Panay N. The REVIVE (REal Women's VIews of Treatment Options for Menopausal Vaginal ChangEs) survey in Europe: country-specific comparisons of postmenopausal women's perceptions, experiences and needs. Maturitas. 2016; 91:81–90.

9. Nappi RE, Particco M, Biglia N, Cagnacci A, Di Carlo C, Luisi S, et al. Attitudes and perceptions towards vulvar and vaginal atrophy in Italian post-menopausal women: evidence from the European REVIVE survey. Maturitas. 2016; 91:74–80.

10. Bachmann G. Urogenital ageing: an old problem newly recognized. Maturitas. 1995; 22:Suppl. S1–S5.

11. Lee D, Kim TH, Lee HH, Kim JM, Jeon DS, Kim YS. A pilot study of the impacts of menopause on the anogenital distance. J Menopausal Med. 2015; 21:41–46.

12. Meston CM. Validation of the female sexual function index (FSFI) in women with female orgasmic disorder and in women with hypoactive sexual desire disorder. J Sex Marital Ther. 2003; 29:39–46.

13. Wiegel M, Meston C, Rosen R. The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005; 31:1–20.

14. Welsh M, Saunders PT, Fisken M, Scott HM, Hutchison GR, Smith LB, et al. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J Clin Invest. 2008; 118:1479–1490.

15. Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, et al. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci. 2000; 58:339–349.

16. Gray LE Jr, Wilson VS, Stoker T, Lambright C, Furr J, Noriega N, et al. Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals. Int J Androl. 2006; 29:96–104. discussion 5-8.
17. Salazar-Martinez E, Romano-Riquer P, Yanez-Marquez E, Longnecker MP, Hernandez-Avila M. Anogenital distance in human male and female newborns: a descriptive, cross-sectional study. Environ Health. 2004; 3:8.

18. Wilson VS, Blystone CR, Hotchkiss AK, Rider CV, Gray LE Jr. Diverse mechanisms of anti-androgen action: impact on male rat reproductive tract development. Int J Androl. 2008; 31:178–187.

19. Mendiola J, Stahlhut RW, Jorgensen N, Liu F, Swan SH. Shorter anogenital distance predicts poorer semen quality in young men in Rochester, New York. Environ Health Perspect. 2011; 119:958–963.

20. Pasterski V, Acerini CL, Dunger DB, Ong KK, Hughes IA, Thankamony A, et al. Postnatal penile growth concurrent with mini-puberty predicts later sex-typed play behavior: evidence for neurobehavioral effects of the postnatal androgen surge in typically developing boys. Horm Behav. 2015; 69:98–105.

21. Thankamony A, Lek N, Carroll D, Williams M, Dunger DB, Acerini CL, et al. Anogenital distance and penile length in infants with hypospadias or cryptorchidism: comparison with normative data. Environ Health Perspect. 2014; 122:207–211.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download