Abstract
Agenesis of the gallbladder and cystic duct represents one of the rarest anomalies of the biliary system, with a reported incidence of 0.007% to 0.027%. Almost half of the patients develop common duct stones and 23% of them manifest signs and symptoms that mimic biliary colic. We present the case of a woman presenting with symptoms of biliary colic. Based on the clinical findings and after abdominal ultrasonography, which showed hyperechoic material in the gallbladder fossa, a laparoscopic cholecystectomy was planned. Laparoscopy failed to reveal either gallbladder or cystic duct. The procedure was continued to further search for ectopic sites of gallbladder. A condition of gallbladder agenesis was hypothesized and the procedure was aborted without dissection of hepatic pedicle or conversion to laparotomy. Agenesis of gallbladder and cystic duct was confirmed via pos-operative magnetic resonance cholangiopancreatography. We report our experience with regard to the challenges associated with the diagnosis and management, and a brief review of the literature of this rare pathology.
Gallbladder agenesis (GA) is a rare congenital condition with a reported incidence of 0.007–0.027% in surgical series (0.04–0.13% in autopsy series). It is estimated up to 50% of patients will develop common duct stones at some point, and 23% will eventually manifest the clinical symptoms suggestive of gallbladder disease.1
This rare anomaly originates at the end of the first month of life in utero, when the cystic bud develops from the caudal part of the foregut.2 It may be occasionally associated with other anatomical congenital anomalies or congenital syndromes such as cerebrotendinous xanothomatosis, Klippel-Feil syndrome, trisomy 18, and following exposure to thalidomide.3
To date, only approximately 400 cases of GA are reported in literature.4 Routine investigations frequently fail to diagnose GA. Usually the diagnosis is established incidentally during laparotomy or laparoscopy in patients presenting with signs and symptoms that mimic a biliary colic or cholelithiasis.
We performed a brief review of the literature to high-light the diagnostic and management challenges of this rare pathology.
A 45-year-old Caucasian woman presented to “A. Gemelli” Teaching Hospital of Rome, a 1200-bed tertiary medical center, with bloating symptoms and frequent dyspepsia. Her medical history included hypertension while the surgical history included appendectomy at the age of 10 years. No congenital syndromes or anomalies were reported. She suffered two episodes of pain involving the upper-right abdominal quadrant associated with nausea after fatty food meal, at 6 and 2 weeks, respectively, prior to recovery, and was successfully treated with NSAIDs and smooth muscle relaxants. An abdominal ultrasound (US) showed moderate-to-severe hepatosteatosis and hepatomegaly. Gallbladder was not visualized adequately, especially its wall, however, a hyperechoic material with acoustic shadow was detected in the usual place of gallbladder. The common bile duct was not dilated. Clinical examination was normal except for tenderness in the right upper quadrant of the abdomen with mild pain rebound following percussion. Routine laboratory data were in the normal range except for gamma-glutamyltransferase (78 U/L, normal value 5-36). We made a diagnosis of cholelithiasis and performed a laparoscopic cholecystectomy.
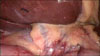
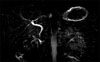
We placed a 10-mm infraumbilical port and three additional 5-mm trocars in epigastrium, right flank and mesogastrium, respectively. The liver was enlarged with a big left hemi-liver and the transverse colon appeared dilated. Neither gallbladder nor gallbladder fossa or cystic duct was identified upon lifting the liver (Fig. 1). We analyzed the abdomen searching for potential sites of ectopic gallbladder (such as between the leaves of the lesser omentum, within the falciform ligament or in the retroduodenal areas), and failed to detect any such ectopic site. Therefore, a condition of GA was hypothesized. No apparent anatomical abnormalities of hepatic pedicles or viscera were found, and therefore, the procedure was interrupted. A subhepatic drainage was placed. Postoperative course was uneventful and the drainage was removed the day after. Patient then underwent magnetic resonance cholangiopancreatography (MRCP, Fig. 2), which confirmed the absence of the gallbladder (Type 3 according to Bennion classification,5 Subtype Ib according to LM Tang classification).6 The extra-hepatic and intra-hepatic bile ducts were normal. The only other anomaly recognized was the anatomy of the pancreatic duct, suggesting a possible pancreas divisum, which is often related to GA. We discontinued any further investigation in the absence of symptoms or stones following MRCP, or anatomical variation. The hyperechoic mass detected in the abdominal US correlated with colonic content. Patient was successively discharged with dietary recommendation and is free of symptoms after 12 months.
The gallbladder originates in the cystic bud, which develops as a ventral outgrowth from the caudal region of the foregut in the fourth week of life in utero.2 During the seventh week of its development, vacuolation of the hyperplastic epithelium occurs. The gallbladder and cystic duct develop a lumen. It was hypothesized that an anomaly at any stage of this process results in agenesis of the gallbladder,7 occasionally associated with other congenital malformations such as extrahepatic bile duct atresia, imperforate anus, cardiovascular anomalies, intestinal malrotation, and so forth.3 Inappropriate migration of gallbladder during embryonic development leads to the formation of ectopic gallbladder, generally intra-hepatic and left-sided, between the leaves of lesser omentum, within the falciform ligament, retropancreatic and retroduodenal.8
The different clinical presentations of GA reflect the variety of embryological anomalies and were classified by Bennion et al.5 into three categories: 1) association with other congenital malformations often leads to perinatal death (12.8–30%); 2) asymptomatic cases (31.6%); and 3) symptomatic patients (55.6 %). Recently Tang et al.6 proposed a new classification including symptomatic subtype Ia GA accompanied by lethal deformities such as biliary atresia, imperforate anus, ventricular septal defect, and duodenal atresia (the majority of these patient die shortly after birth); and subtype Ib, which is accompanied by non-lethal deformities such as intestinal malrotation, right liver agenesis, cryptorchidism, and choledochal cyst.
Symptomatic patients usually complain of right upper quadrant pain (90%), nausea and/or vomiting (66%), fatty food intolerance (37.5%), dyspepsia, bloating, or jaundice occasionally.5 These symptoms, which are similar to other common biliary tract conditions, are often explained by biliary dyskinesia (retrograde contraction of the sphincter of Oddi), extrabiliary symptoms or primary choledocholithiasis.9
Abdominal US has a reported sensitivity of 95% in gallstone diagnosis even if it depends on factors such as the surgical skill and the examination conditions. Moreover, ultrasound abnormalities decrease the sensitivity to 61%.10 Frequently in these patients, abdominal US fails to diagnose GA due to gas artifact, periportal tissue or subhepatic peritoneal folds,11 leading to a wrong diagnosis of shrunken or contracted gallbladder.12 Generally anomaly of the WES triad (Wall visualization, Echo of a stone and acoustic Shadow), should raise the suspect of GA.13
These clinical features, together with the ignorance of medical staff and the rarity of GA among the anomalies of the biliary tract, contribute to diagnostic difficulty. Most of the cases reported in the literature received an intraoperative diagnosis since abdominal US often fails to demonstrate this condition preoperatively. No consensus is available on the appropriate management of patients in the absence of gallbladder during surgery, due to the paucity of data reported. A few experts recommend conversion to laparotomy during surgical procedure to facilitate an accurate investigation of heterotopic sites of gallbladder.6 Other investigators proposed intraoperative cholangiograhy or invasive procedure of the bile duct1714 while others discourage these approaches due to possible complications and recommend further radiological investigation.115
A safe and noninvasive GA diagnosis of GA is facilitated by a computed tompgraphy scan, an endoscopic retrograde cholangiopancreatography (ERCP) (diagnostic and eventually operative in case of choledocolithiasis) or an MRCP without contrast enema, and is not affected by biliary stasis.1617 MRCP is considered the gold standard for suspected GA, and in demonstrating an ectopic gallbladder or other possible anomalies of the biliary tract.18 Laparoscopic or endoscopic US is effective in GA diagnosis but its poor availability limits its utility in hepatobiliaryscintigraphy scans.19
GA is managed conservatively with smooth muscle relaxant and sphincterotomy.
Almost all of our colleagues arrived at an incorrect preoperative diagnosis, even after subjecting the patient to further radiological investigation.1720 Despite the inconsistencies detected in the abdominal US, a preoperative diagnosis was established only in a few cases without the need for surgery.16
In conclusion, this rare condition should be borne in mind when the gallbladder is not well recognized in routine US. We hypothesize that exploratory laparoscopy may play a diagnostic role in patients with uncertain findings in instrumental tests or in patients contraindicated for MRCP or ERCP. However, we recommend neither an intraoperative dissection of the hepatic pedicle nor conversion to an open procedure due to the possible complications associated with these interventions.
Figures and Tables
References
1. Peloponissos N, Gillett M, Cavin R, Halkic N. Agenesis of the gallbladder: a dangerously misdiagnosed malformation. World J Gastroenterol. 2005; 11:6228–6231.
2. Blechschmidt CM. Agenesis of the gallbladder-borderline case of normality? Anat Anz. 1982; 151:281–285.
3. Turkle SB, Swanson V, Chandrasoma P. Malformations associated with congenital absence of the gallbladder. J Med Genet. 1983; 20:445–449.
4. Joliat GR, Shubert CR, Farley DR. Isolated Congenital agenesis of the gallbladder and cystic duct: report of a case. J Surg Educ. 2013; 70:117–120.

5. Bennion RS, Thompson JE Jr, Tompkins RK. Agenesis of the gallbladder without extrahepatic biliary atresia. Arch Surg. 1988; 123:1257–1260.

6. Tang LM, Wang XF, Ren PT, Xu GG, Wang CS. The diagnosis of gallbladder agenesis: two cases report. Int J Clin Exp Med. 2015; 8:3010–3016.
7. Gotohda N, Itano S, Horiki S, Endo A, Nakao A, Terada N, et al. Gallbladder agenesis with no other biliary tract abnormality: report of a case and review of the literature. J Hepatobiliary Pancreat Surg. 2000; 7:327–330.

9. Toouli J, Geenen JE, Hogan WJ, Dodds WH, Arndorfer RC. Sphincter of oddi motor activity: a comparison between patients with common bile duct stones and controls. Gastroenterology. 1982; 82:111–117.

10. Crade M, Taylor KJ, Rosenfield AT, de Graaff CS, Minihan P. Surgical and pathologic correlation of cholecystosonography and cholecystography. AJR Am J Roentgenol. 1978; 131:227–229.

11. Serour F, Klin B, Strauss S, Vinograd I. False-positive ultrasonography in agenesis of the gallbladder: a pitfall in the laparoscopic cholecystectomy approach. Surg Laparosc Endosc. 1993; 3:144–146.
12. Jackson RJ, McClellan D. Agenesis of the gallbladder: a cause of false-positive ultrasonography. Am Surg. 1989; 55:36–40.
14. Mittal AK, Saxena D, Kadam R, Garg P, Kankaria J, Jenaw R. Agenesis of gallbladder: a diagnostic dilemma. Int J Case Rep Images. 2015; 6:296–300.

15. Cabajo CM, Martin del Olmo JC, Blanco AJ, Atienza SR. Gallbladder and cystic duct absence: an infrequent malformation in laparoscopic surgery. Surg Endosc. 1997; 11:483–484.
16. Fiaschetti V, Calabrese G, Viarani S, Bazzocchi G, Simonetti G. Gallbladder agenesis and cystic duct absence in an adult patient diagnosed by magnetic resonance cholangiography: report of a case and review of the literature. Case Rep Med. 2009; 2009:674768.

17. Malde S. Gallbladder agenesis diagnosed intra-operatively: a case report. J Med Case Rep. 2010; 4:285.

18. Pierro A, Martucci M, Maselli G, Farchione A. Agenesis of the gallbladder with the presence of a small dysmorphic cyst: role of magnetic resonance cholangiopancreatography. J Clin Imaging Sci. 2012; 2:17.

19. Gad MA, Krishnamurthy GT, Glowniak JV. Identification and differentiation of congenital gallbladder abnormality by quantitative technetium-99m IDA cholescintigraphy. J Nucl Med. 1992; 33:431–434.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download