Abstract
The incidence of combined hepatocellular carcinoma-cholangiocarcinoma (cHCC-CC) in a single patient accounts for only 0.4 to 14% of all primary liver cancer. However, the prognosis of its intrahepatic cholangiocarcinoma (ICC) component is poor. We experienced a unique case of a sequentially developed cHCC-CC with adrenal metastasis as the primary presentation and a hidden primary hepatocellular carcinoma. A 65-year-old female with a history of jaundice and abdominal discomfort was diagnosed with S4 ICC measuring 5 cm in diameter, and characterized histologically as papillary adenocarcinoma with intraductal growth, but without any evidence of malignant hepatocyte. S4 segmentectomy with hepaticojejunostomy revealed no additional masses. A follow-up CT scan 3 months after surgery showed a right adrenal mass with markedly increased serum AFP (4950 ng/mL), which was treated with right adrenalectomy. Histopathology revealed a metastatic hepatocellular carcinoma testing positive for AFP, glypican-3, and hepatocytes, but negative for CD-10, inhibin-α, EMA, S-100, and cytokeratin-7. Serum AFP level immediately plummeted to 4.1 ng/mL upon adrenal mass removal. A recurrent S7 liver mass was suspected 1 year later with serum AFP value of 7.6 ng/mL, and characteristic CT imaging of HCC. TACE was performed with good response. Adrenal metastasis may manifest as the primary focus of hepatocellular carcinoma in sequentially developed cHCC-CC patients with hidden primary HCC. cHCC-CC should be considered in the differential diagnosis of cholangiocarcinoma with elevated AFP.
A combined hepatocellular carcinoma-cholangiocarcnoma is a relatively rare disease entity, accounting for only 0.4–14% of all primary liver cancer.1234 The classification of hepatocellular carcinoma-cholangiocarcnoma first developed by Allen Lisa in 1949 consisted of 3 subtypes: 1) type A, characterized by synchronous, separate and autonomous epicenters of hepatocellular carcinoma (HCC) and cholangiocarcnoma (CC) in a single liver; 2) type B, comprising closely admixed and distinguished foci of HCC and CC; and 3) type C, consisting of truly combined HCC and CC components originating in the same tumor.15
Surgical resection may still lead to intra- and extra-hepatic recurrence, which is a major concern. Although relatively common in hepatocellular carcinoma (HCC), metastasis to adrenal gland rarely occurs in intrahepatic cholangiocarcinoma (ICC) and combined HCC-CC (cHCC-CC). We experienced a unique case of sequentially developed cHCC-CC, classified as Allen Lisa A, with adrenal metastasis as the primary presentation and a hidden primary HCC.
Sixty-five years old female with a history of jaundice and abdominal discomfort with a high serum alpha-fetoprotein (AFP) and low serum chorioembryonic antigen (CEA) (532 ng/ml and 1.9 mg/dl, respectively). No evidence of prior hepatitis infection was found and preoperative computed tomography (CT) scans showed multiple enhancing lesions intraductally within the left intrahepatic duct as shown in Fig. 1. She was preoperatively diagnosed with segment 4 mixed type cHCC-CC (Allen Lisa C), and underwent an S4 segmentectomy and hepaticojejunostomy. No additional masses were found during surgery. As illustrated in Fig. 2, the pathological result confirmed a 5-cm papillary adenocarcinoma, with intraductal growth, without any evidence of malignant hepatocytes.
A follow-up CT scan for disease surveillance revealed a right adrenal mass that gradually enlarged, with a marked increase in serum AFP (4,950 ng/mL). Magnetic resonance imaging (MRI) and positron emission tomography (PET)-CT also suggested a metastatic lesion in the right adrenal gland (Fig. 3). A percutaneous adrenal gland biopsy confirmed a metastatic HCC (positive for AFP, glypican-3, and hepatocytes, and negative for CD-10, inhibin-α, EMA, S-100, and cytokeratin-7), and right adrenalectomy was performed uneventfully. The specimen is shown in Fig. 4. As shown in Fig. 5, the serum AFP immediately declined to 4.1 ng/mL, shortly after adrenalectomy. Unfortunately, a recurrent S7 liver mass was suspected 1 year after adrenalectomy, with serum AFP 7.6 ng/mL and CT imaging characteristic of HCC. A series of Trans-arterial chemo-embolization (TACE) and RadioFrequency Ablation (RFA) procedures were then performed with favorable responses (Fig. 6).
cHCC-CC was first reported by Wells in 1903, and further classified by Allen and Lisa in 1949 and Goodman in 1985.56 It originates in hepatic progenitor cells, the liver-specific bipotential adult stem cells, which are activated upon injury to hepatocytes and/or cholangiocytes.7 Thus, it has the ability to differentiate into hepatocellular carcinoma, cholangiocarcinoma, or both. A few ductal plate malformations, particularly von-Meyenburg complex, show malignant transformation to intrahepatic CC (ICC) suggesting the possibility of combined HCC and ICC originating in ductal plate malformation.8
Histophatologic diagnosis of cHCC-CC, according to World Health Organization (WHO), is characterized by an unequivocally differentiated hepatocellular and biliary component in one tumor, which correspondends to Allen Lisa C (Mixed Type) or Goodman I (Collision tumor).1 Accurate preoperative diagnosis is difficult and often leads to misdiagnosis. cHCC-CC should be highly suspected in cases where imaging reveals both HCC and CC features, regardless of tumor marker levels; in the presence of elevated tumor markers (AFP and CA19-9, regardless of imaging features) or conflicting imaging and tumor marker findings.
Initial preoperative diagnosis in our case report was cHCC-CC, based on the contradictory findings: tumor marker level (a very high AFP) and imaging features of cholangiocarcinoma (intraductal gradually enhancing lesion within the left intrahepatic duct). However, since no additional metastatic lesions were present, liver resection and lymph node dissection was a rational choice,13 despite the fact that the recurrence rate after surgery was still higher than in hepatocellular carcinoma (disease-free survival of HCC vs. cHCC vs. ICC : 68.2 months vs 23.4 months vs 15.5 months).9
Several prognostic factors of cHCC-CC associated with unfavorable outcome include large tumor size, vascular invasion, lymph node metastasis, and the presence of satellite lesions.69 Ariizumi et al.10 reported a median survival of 15.4 months, with a 5-year overall survival rate found in 24% of all patients. Another report by Lee at al.9 showed an overall survival rate of 47.3 months, shorter than HCC (71.7 months), but longer than ICC (21.5 months).
With regards to the nature of disease recurrence and poor survival, close surveillance after surgery is an important aspect of management strategy, as in HCC or ICC. Early intra- and extra-hepatic recurrence was detected, warranting regular imaging surveillance as well as tumor marker follow-up studies. The incidence of extra-hepatic metastasis is around 13.5 to 42 %,1112 including metastasis to adrenal glands (12%).11 Presentation of a cholangiocellular component appeared to be a poor prognostic indicator.13
Since both adrenal glands are almost always included in the scanning area, any changes in size and glandular characteristics may be visualized to detect adrenal incidentalinoma, 3 an adrenal tumor discovered fortuitously during imaging for other indications.14 Possibility of malignancy increases with large tumor size, heterogeneous lesion and presence of peritoneal lymphadenopathy. Because of the rich sinusioidal blood flow and its multiple arterial supplies, the adrenal gland is a potential site for metastasis.15 The mechanism of extrahepatic metastasis from primary liver tumor to adrenal glands may involve a direct extension through exophytic growth or systemically through retroperitoneal venous system.12 Poor differentiation of malignant lesions from benign adenoma remains a diagnostic challenge in the management of adrenal incidentalinoma.16
The diagnosis of adrenal metastasis in our case was straightforward—based on the size of the lesion, history of previous malignancy, and low attenuation in pre-contrast study. However, percutaneous biopsy was indicated for accurate histopathological diagnosis, due to conflicting results based on initial preoperative diagnosis of cHCC-CC and postoperative histopathology of ICC.
The 5-year survival rate of HCC patients with resected adrenal metastasis was reported around 20% to 45%.15 When compare to other modality or no treatment, adrenalectomy provides the highest survival (11.1 months, 5.7 months, 21.4 months, respectively).17 Disease-free interval of less than 12 months is associated with poor survival.15
Interestingly, we detected a sequentially developed cHCC-CC (Allen Lisa A), with adrenal metastasis as the first presentation of a hidden hepatocellular carcinoma. Although S4 mass was cholangiocarcinoma, metastasis or recurrent mass showed HCC-like features. Tsalis et al.18 first reported the case of adrenal metastasis as the first presentation of HCC in a 76-year-old male with a marked increase in tumor markers but without initial liver masses. Pandey et al.19 reported a case of adrenal metastasis from ICC, and the patient is still alive with recurrent disease 2 years after surgery.
The sequentially developed cHCC-CC in this case was unique and invaluable although the S4 mass was cholangiocarcinoma, whereas metastatic recurrent mass showed HCC-like figures. Our report suggests that a markedly increased level of tumor markers, after resection of a liver malignant lesion, without a visible postoperative liver mass, requires comprehensive evaluation for the possibility of disease recurrence. In conclusion, adrenal metastasis may present as the primary focus of HCC in sequentially developed cHCC-CC patient with hidden primary HCC.
Figures and Tables
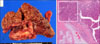
Fig. 1
Initial preoperative CT scan showing a multiple intra-ductal gradually enhancing lesions within the left intrahepatic duct.

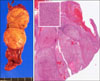
Fig. 2
Liver S4 resection specimen with intraductal tumor infiltration suggesting intrahepatic cholangiocarcinoma.
References
1. Maximin S, Ganeshan D, Shanbhogue A, Dighe MK, Yeh MM, Kolokythas O, et al. Current update on combined hepatocellular-cholangiocarcinoma. Eur J Radiol Open. 2014; 1:40–48.

2. Yap AQ, Chen CL, Yong CC, Kuo FY, Wang SH, Lin CC, et al. Clinicopathological factors impact the survival outcome following the resection of combined hepatocellular carcinoma and cholangiocarcinoma. Surg Oncol. 2013; 22:55–60.
3. Park JS, Yoon DS, Kim KS, Choi JS, Lee WJ, Chi HS, et al. What is the best treatment modality for adrenal metastasis from hepatocellular carcinoma? J Surg Oncol. 2007; 96:32–36.

4. O'Connor K, Walsh JC, Schaeffer DF. Combined hepatocellular-cholangiocarcinoma (cHCC-CC): a distinct entity. Ann Hepatol. 2014; 13:317–322.
5. Allen RA, Lisa JR. Combined liver cell and bile duct carcinoma. Am J Pathol. 1949; 25:647–655.
6. Yap AQ, Chen CL, Yong CC, Kuo FY, Wang SH, Lin CC, et al. Clinicopathological factors impact the survival outcome following the resection of combined hepatocellular carcinoma and cholangiocarcinoma. Surg Oncol. 2013; 22:55–60.
7. Akiba J, Nakashima O, Hattori S, Tanikawa K, Takenaka M, Nakayama M, et al. Clinicopathologic analysis of combined hepatocellular-cholangiocarcinoma according to the latest WHO classification. Am J Surg Pathol. 2013; 37:496–505.

8. Terada T. Combined hepatocellular-cholangiocarcinoma ith stem cell features, ductal plate malformation subtype: a case report and proposal of a new subtype. Int J Clin Exp Pathol. 2013; 6:737–748.
9. Lee WS, Lee KW, Heo JS, Kim SJ, Choi SH, Kim YI, et al. Comparison ofcombined hepatocellular and cholangiocarcinoma with hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Surg Today. 2006; 36:892–897.
10. Ariizumi S, Kotera Y, KatagiriS , Nakano M, Yamamoto M. Combined hepatocellular-cholangiocarcinoma had poor outcomes after hepatectomy regardless of Allen and Lisa class or the predominance of intrahepatic cholangiocarcinoma cells within the tumors. Ann Surg Oncol. 2012; 19:1628–1636.
11. Yasaka K, Gonoi W, Akai H, Katsura M, Akahane M, Kiryu S, et al. Differentiation of adrenal tumors in patients with hepatocellular carcinoma: adrenal adenoma versus metastasis. Eur J Radiol. 2013; 82:1213–1218.

12. Chua TC, Morris DL. Exploring the role of resection of extrahepatic metastasis from hepatocellular carcinoma. Surg Oncol. 2012; 21:95–101.
13. Park H, Choi KH, Choi SB, Choi JW, Kim DY, Ahn SH, et al. Clinicopathological characteristics in combined hepatocellular-cholangiocarcinoma: a single center study in Korea. Yonsei Med J. 2011; 52:753–760.

14. Germain A, Klein M, Brunaud L. Surgical management of adrenal tumors. J Visc Surg. 2011; 148:e250–e261.

15. Sancho JJ, Triponez F, Montet X, Sitges-Serra A. Surgical management of adrenal metastases. Langenbecks Arch Surg. 2012; 397:179–194.

16. Birsen O, Akyuz M, Dural C, Aksoy E, Aliyev S, Mitchell J, et al. A new risk stratification algorithm for the management of patients with adrenal incidentalomas. Surgery. 2014; 156:959–965.

17. Ha TY, Hwang S, Ahn C, Kim KH, Lee YJ, Moon DB, et al. Resection of metachronous adrenal metastasis after liver resection and transplantation for hepatocellular carcinoma. Dig Surg. 2014; 31:428–435.

18. Tsalis K, Zacharakis E, Sapidis N, Lambrou I, Zacharakis E, Betsis D. Adrenal metastasis as first presentation of hepatocellular carcinoma. World J Surg Oncol. 2005; 3:50.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download