Abstract
Backgrounds/Aims
Pancreatic leakage is a major cause of postoperative death and morbidity after pancreaticoduodenectomy (PD). A recent study introduced Blumgart anastomosis (BA), which minimizes severe complications after PD. This study compares BA with conventional anastomosis (CA) for pancreaticojejunostomy (PJ) after PD at a single institution.
Methods
A total of 87 patients who underwent PD at our hospital between January 2003 and October 2015 were enrolled in this study. The patients were divided into two groups according to the anastomosis type. Of them, 44 patients underwent anastomosis using CA (group A, conventional duct-to-mucosa anastomosis) and 43 underwent anastomosis using BA (group B, Blumgart anastomosis).
Results
There was a significant difference in duration of the operation between groups A and B (473.1±102.0 versus 386.4±58.5 min, p<0.001) and intraoperative transfusion (2.2±2.7 versus 0.7±1.5 units, p<0.001). There was no significant difference between groups A and B in incidence of postoperative pancreatic fistula (POPF) (43.2% versus 27.9%, p=0.137) ,postoperative hemorrhage (PPH) (13.7% versus 7.0%, p=0.209), delayed gastric emptying (DGE) (29.5% versus 9.3%, p=0.063), surgical and non-surgical complications (60.5% versus 59.1%, p=0.896), length of ICU stay (9.0±6.3 versus 7.4±7.2 days, p=0.099), or length of postoperative hospital stay (37.7±16.7 versus 41.6±15.1 days, p=0.118).
Pancreaticoduodenectomy (PD) is a surgical procedure for malignant and benign tumors of the pancreatic head, common bile duct, duodenum, and ampulla of Vater. Pancreaticoenterostomy after PD is called “Achilles' heel” because it entails the highest risk of surgical complications among all abdominal anastomoses, resulting in pancreatic fistula (PF).1 PF is a major cause of postoperative complications after PD and a potentially serious, lifethreatening event associated with intra-abdominal fluid collection or abscess, hemorrhage, the occasional need for reoperation, and possible death.2 At high-volume hospitals, deaths after pancreatic resection have recently decreased to less than 5%, but the morbidity remains high, ranging from 30% to 50%.3 Although various techniques of pancreaticojejunostomy (PJ) have been attempted to decrease postoperative deaths and morbidity, there is still no standard technique to decrease the incidence of pancreatic leakage.4
In 2000, a new technique was first described by Blumgart. The technique includes duct-to-mucosa anastomosis and four transpancreatic U-sutures. Recently, Kleespies et al.5 reported that Blumgart anastomosis (BA) minimizes severe complications after PD. Our study was conducted to compare BA with conventional anastomosis (CA) for PJ after PD.
A total of 87 patients who underwent PD at the Department of Surgery, Presbyterian Medical Center, between January 2003 and October 2015 were enrolled in this study. CA was exclusively used before 2010, and BA was used after 2010. The patients were divided into two groups according to the anastomosis type: those who underwent CA (group A, n=44) and those who underwent BA (group B, n=43). Patient characteristics, surgical parameters, postoperative complications, and deaths were compared between the two groups. This study was approved by the Institutional Review Board of Presbyterian Medical Center.
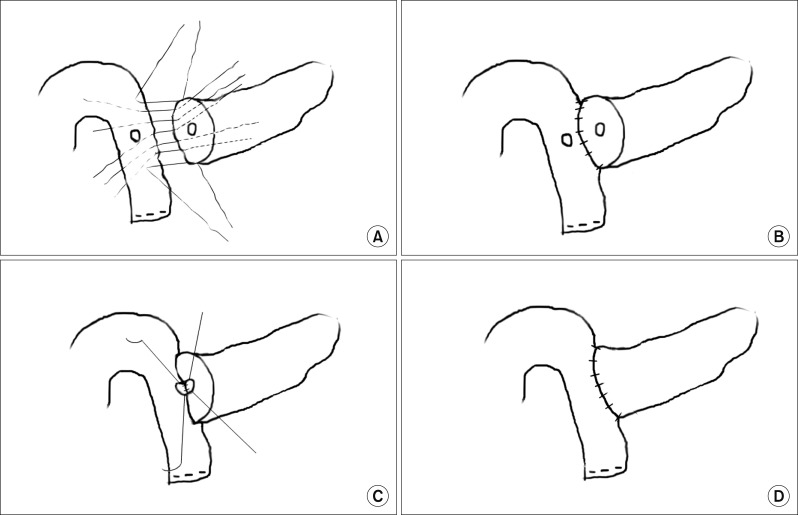
CA is a duct-to-mucosa PJ (Fig. 1). Six to eight non-absorbable interrupted sutures (SoftsilkTM3-0, Covidien) were placed between the posterior surface of the pancreas and the seromuscular layer of the jejunum. After making a small incision in the jejunum, four to six absorbable interrupted sutures (Vicryl 5-0, Ethicon) were placed between the pancreatic duct and the jejuna mucosa in an end-to-side fashion, and then the anterior layer of the pancreas and the jejunum was approximated in the same manner as described above.
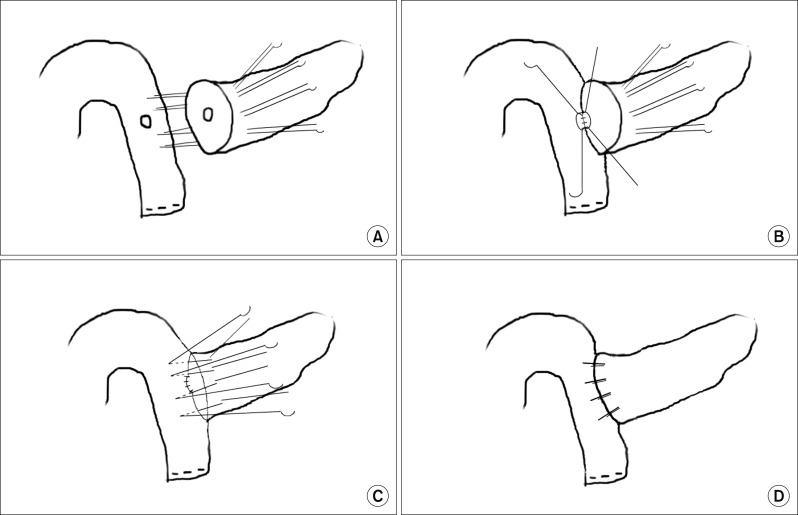
BA is a new transpancreatic U-suture technique with modification of duct-to-mucosa anastomosis (Fig. 2). It was originally introduced by Dr. Leslie Blumgart and reported by Grobmyer et al.6 at Memorial Sloan Kettering Cancer Center. This technique includes placement of four to six transpancreatic sutures and jejunalseromuscular sutures to approximate the pancreas and the jejunum using non-absorbable sutures (SoftsilkTM2-0, Covidien). A suture was placed through the whole pancreatic parenchyma from front to back. A seromuscular bite with vertical mattress over the jejunum was taken as the posterior outer layer, and the same suture reverted back to front through the whole pancreas again to complete the U-suture. These transpancreatic U-sutures were completed by placing the needle on the anterior seromuscular wall of the jejunum to create an anterior outer layer after constructing duct-to-mucosa anastomosis.5 Then, the pancreatic remnant was totally covered with jejunal serosa. Pancreatic duct stents were not routinely inserted, but were used at the discretion of the surgeon.
Major complications after PD included postoperative pancreatic fistula (POPF), postpancreatectomy hemorrhage (PPH), and delayed gastric emptying (DGE). POPF was defined and graded according to the International Study Group on Pancreatic Fistulas (ISGPF).3 The ISGPF proposed the clinical grading system of POPF by severity (grades A, B, and C), with grade A being least severe and grade C being most severe.3 This grading system of POPF was based on parameters, such as clinical condition, treatment used, imaging study results, persistent drainage, reoperation, death, infection signs, and readmission.3 PPH and DGE were defined and graded according to the International Study Group on Pancreatic Surgery (ISGPS).78 Three different grades of PPH (grades A, B, and C) were defined according to the time of onset, site of bleeding, severity, and clinical effect.7 The mild, moderate, and severe forms of DGE after pancreatic resection were classified into grades A, B, and C, respectively, based on their clinical effect on the clinical course and on postoperative management.8 Intra-abdominal abscess or fluid collection was detected by postoperative computed tomography (CT) scans. Non-surgical complications were defined as a Clavien-Dindo score III or higher.
Comparisons between the two groups were made using the Mann-Whitney U test for continuous variables and the χ2 test and Fisher's exact test for categorical variables, which are presented as frequency or percentage. Continuous variables are presented as mean±SD. Statistical significance was set at p<0.05. Statistical data were calculated by SPSS version 23.0 (IBM SPSS Statistics, IBM Corporation, Chicago, IL, USA).
A total of 87 patients were included in the present study. Of these, 44 underwent CA (group A) and 43 patients underwent BA (group B). The clinical and demographic characteristics of patients in both groups are shown in Table 1. There was no significant difference in the clinical and demographic characteristics except for preoperative intervention and body mass index (BMI) between the two groups. Preoperative intervention was performed more frequently in group B than in group A. Especially, patients in group B more frequently underwent endoscopic retrograde biliary drainage or endoscopic nasobiliary drainage than did group A (69.8% versus 38.6%, p=0.004). BMI was higher in group B than in group A (24±3 versus 22±2, p=0.001). The incidence of hypertension was also higher in group B than in group A (20.5% versus 55.8%, p<0.01).
Data about the surgical procedures are shown in Table 2. There was a significant difference between the two groups in the incidence of malignant and benign diseases. In group B, there were 38 patients (88.4%) with malignant disease and two (4.7%) with benign disease. In group A, there were 36 patients (81.8%) with malignant disease and eight (18.2%) with benign disease in the pancreatic head. The proportion of malignant disease was higher in group B than in group A, whereas that of benign disease was higher in group A than in group B (p=0.033)
There were significant differences in median duration of operation and in intraoperative transfusion between the two groups. Median duration of operation was significantly shorter in group B (473.1±102.0 versus 386.4±58.5 min, p<.001), and the number of intraoperative transfusion units was significantly smaller in group B (2.2±2.7 versus 0.7±1.5 unit, p<.001). The lengths of ICU stay and postoperative hospital stay were not statistically different between groups B and A (9.0±6.3 versus 7.4±7.2 days, p=0.099 and 37.7±16.7 versus 41.6±15.1 days, p=0.118, respectively).
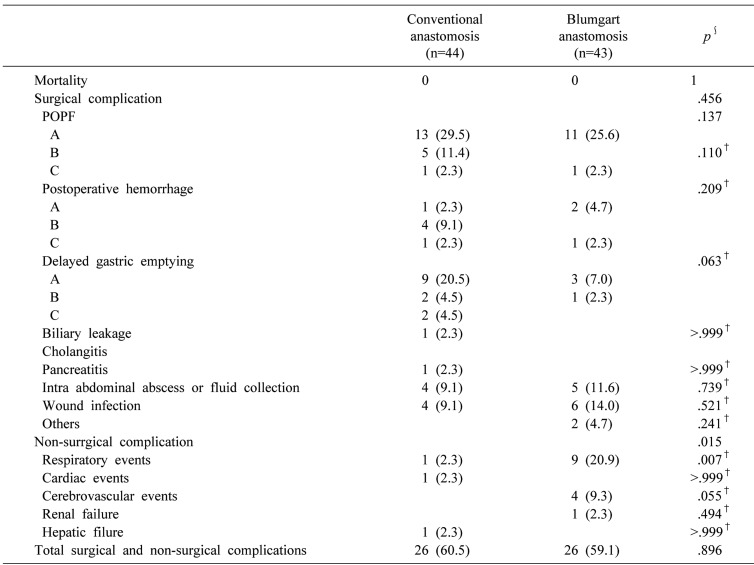
Postoperative complications are listed in Table 3. A significant difference was found in the incidence of non-surgical complications between the two groups. Respiratory complications more frequently occurred in group B. There were no significant differences between groups B and A in surgical complications including POPF (43.2% versus 27.9%, p=0.137), POPF grades B and C (13.7% versus 2.3%, p=0.110), PPH (13.7% versus 7.0%, p=0.209), or DGE (29.5%versus 9.3%, p=0.063). There was no significant difference in the overall incidence of surgical and non-surgical complications between groups B and A (60.5% versus 59.1%, p=0.896). There were no deaths in either group.
Deaths after PD is low, but morbidity remains high. The main postoperative complications include POPF, PPH, and DGE. POPF is a major cause of morbidity after PD. Several surgical techniques have been studied to prevent pancreatic fistulae. However, there is still no standard technique to prevent POPF. We have observed healing of POPF with internal drainage after enucleation of pancreatic benign tumors. Thus, a PJ-site fistula is expected to properly heal with internal drainage, if the sutures between the jejunum and the pancreas firmly anchor the gland to the jejunum, preventing full disruption of pancreaticojejunostomy. In order to prevent tangential tension and shear forces during the healing of POPF, we decided to make the fewest mattress sutures. Based on these results, BA-type PJ was thought to be a suitable procedure and has been performed since 2010 in our department.
There are many types of pancreaticoenterostomies, including PJ and pancreaticogastrostomy. Additionally, there are many types of PJ, such as duct-to-mucosa anastomosis, an invagination-like “dunking” technique, and various modifications. PJ is generally the preferred technique for reconstruction after PD. However, Bassi et al.9 indicated that pancreaticogastrostomy reduces the risk of associated complications, including biliary fistula, postoperative fluid collection, and DGE. Topal et al.10 demonstrated that pancreaticogastrostomy decreases the incidence of POPF.
The conventional duct-to-mucosa method is an end-to-side anastomosis technique. Z'graggen et al.11 reported that POPF developed in 2.1% of 331 patients who underwent conventional duct-to-mucosa anastomosis after pancreatic head resection. Some authors have described invagination procedures. Langrehr et al.12 described a new mattress suture technique that was performed after placing an incision of the same size as the pancreatic cut surface in the jejunal loop and positioning U-stitches, which resembles the previously reported “Dunking” technique. They also stated that there was no significant difference in the incidence of POPF between the duct-to-mucosa and new mattress anastomosis groups. Peng et al.13 also proposed that the hospital stay is shortened and the incidence of POPF is decreased (compared to the results from conventional PJ) by binding PJ , in which the distal 3 cm of the jejunal loop to be used for anastomosis is everted and its mucosa is ablated by either electro coagulation or topical treatment with 10% carbolic acid, followed by immediate rinsing in 75% ethanol and normal saline. However, Maggiori et al.14 showed that PPH is more frequent in the binding PJ group after PD.
Several modified techniques have been reported. In a recent study, Kleespies et al.5 reported that BA minimizes severe complications after PD. Grobmyer et al.6 suggested that Blumgart PJ is applicable to all patients in whom the pancreatic duct is identified and is associated with significantly low postoperative morbidity and deaths. Fujii et al.15 documented that the incidence of POPF was lower in the modified Blumgart anastomosis group, which used only one to three sutures, than in the Kakita group.
Our study was designed to compare BA with CA for PJ after PD at a single institution. There was no significant difference in morbidity between the two groups, unlike in other studies. In our study, there was no significant difference in the overall incidence of surgical and non-surgical complications between the two groups. However, significant differences were found in median duration of operation and intraoperative transfusion. Also, POPF, PPH, and DGE developed more frequently in group A, although the difference was not statistically significant.
The International Study Group of Pancreatic Fistula classification was updated during this study; the grade A postoperative pancreatic fistula was called a “biochemical leak,” because it has no clinical significance.16 Therefore, we re-analyzed the incidence of POPF grades B and C. There was no significant difference between groups B and A in the incidence of POPF grades B and C (13.7% versus 2.3%, p=0.110). However, POPF grades B and C occurred more frequently in group A. Further study with more cases would be necessary to improve these results.
Respiratory events occurred more frequently in group B, perhaps because of the changes in therapeutic percutaneous drainage techniques over time or because the group B patients were slightly older than those in group A.
Our study has some limitations. First, it was a retrospective, single-center study. Second, it was based on a small sample size. Third, these patients were not contemporaneous; a difference in technical proficiency may have developed over time.
Despite the small sample size, we recognized that the PJ anastomosis is an important and key procedure. If POPF occurs, we want to protect the main bleeding site, such as the gastroduodenal artery stump, and to do adequate drainage. Owing to delayed hemorrhage from the gastroduodenal artery stump, we have protected the gastroduodenal artery from crushing injury during ligation and cutting of the artery. Additionally, we used duct-to-mucosa anastomosis with six or more stitches and omental patches in the Blumgart anastomosis.
In conclusion, there were significant differences between the two groups in duration of operation and intraoperative transfusion, although the overall incidence of surgical and non-surgical complications was not significantly different. The results of this study suggest that BA-type PJ is not inferior to CA-type PJ in terms of postoperative complications.
References
1. Batignani G, Fratini G, Zuckermann M, Bianchini E, Tonelli F. Comparison of Wirsung-jejunal duct-to-mucosa and dunking technique for pancreatojejunostomy after pancreatoduodenectomy. Hepatobiliary Pancreat Dis Int. 2005; 4:450–455. PMID: 16109535.
2. Berger AC, Howard TJ, Kennedy EP, Sauter PK, Bower-Cherry M, Dutkevitch S, et al. Does type of pancreaticojejunostomy after pancreaticoduodenectomy decrease rate of pancreatic fistula? A randomized, prospective, dual-institution trial. J Am Coll Surg. 2009; 208:738–747. discussion 747-749. PMID: 19476827.

3. Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005; 138:8–13. PMID: 16003309.

4. Schoellhammer HF, Fong Y, Gagandeep S. Techniques for prevention of pancreatic leak after pancreatectomy. Hepatobiliary Surg Nutr. 2014; 3:276–287. PMID: 25392839.
5. Kleespies A, Rentsch M, Seeliger H, Albertsmeier M, Jauch KW, Bruns CJ. Blumgart anastomosis for pancreaticojejunostomy minimizes severe complications after pancreatic head resection. Br J Surg. 2009; 96:741–750. PMID: 19526614.

6. Grobmyer SR, Kooby D, Blumgart LH, Hochwald SN. Novel pancreaticojejunostomy with a low rate of anastomotic failure-related complications. J Am Coll Surg. 2010; 210:54–59. PMID: 20123332.

7. Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007; 142:20–25. PMID: 17629996.
8. Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007; 142:761–768. PMID: 17981197.

9. Bassi C, Falconi M, Molinari E, Salvia R, Butturini G, Sartori N, et al. Reconstruction by Pancreaticojejunostomy versus pancreaticogastrostomy following pancreatectomy: results of a comparative study. Ann Surg. 2005; 242:767–771. PMID: 16327486.
10. Topal B, Fieuws S, Aerts R, Weerts J, Feryn T, Roeyen G, et al. Pancreaticojejunostomy versus pancreaticogastrostomy reconstruction after pancreaticoduodenectomy for pancreatic or periampullarytumours: a multicentre randomized trial. Lancet Oncol. 2013; 14:655–662. PMID: 23643139.
11. Z’graggen K, Uhl W, Friess H, Büchler MW. How to do a safe pancreatic anastomosis. J Hepatobiliary Pancreat Surg. 2002; 9:733–737. PMID: 12658408.
12. Langrehr JM, Bahra M, Jacob D, Glanemann M, Neuhaus P. Prospective randomized comparison between a new mattress technique and cattell (duct-to-mucosa) pancreaticojejunostomy for pancreatic resection. World J Surg. 2005; 29:1111–1119. discussion 1120-1121. PMID: 16091984.

13. Peng SY, Wang JW, Lau WY, Cai XJ, Mou YP, Liu YB, et al. Conventional versus binding pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Ann Surg. 2007; 245:692–698. PMID: 17457161.
14. Maggiori L, Sauvanet A, Nagarajan G, Dokmak S, Aussilhou B, Belghiti J. Binding versus conventional pancreaticojejunostomy: after pancreaticoduodenectomy: a case-matched study. J Gastrointest Surg. 2010; 14:1395–1400. PMID: 20577828.

15. Fujii T, Sugimoto H, Yamada S, Kanda M, Suenaga M, Takami H, et al. Modified blumgart anastomosis for pancreaticojejunostomy: technical improvement in matched historical control study. J Gastrointest Surg. 2014; 18:1108–1115. PMID: 24733259.

16. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017; 161:584–591. PMID: 28040257.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download