Abstract
Backgrounds/Aims
Hilar cholangiocarcinomas (HCCAs) are tumors that involve the biliary confluence; at present, radical surgery offers the only chance of long-term survival, but this can be challenging given the complexity of the hilar anatomy. Blumgart and Jarnagin described a preoperative staging system that incorporates the effect of local tumor extent and its impact on adjacent structures and that has been demonstrated to correlate better with actual surgical resectability. The primary aim of this study is to describe the correlation between preoperative Blumgart-Jarnagin staging and its correlation with surgical resectability.
Methods
Patients who underwent surgical resection for hilar cholangiocarcinoma at Singapore General Hospital between January 1, 2002, and January 1, 2013, were identified from a prospectively maintained institutional database. All patients were staged based on the criteria described by Blumgart and Jarnagin. Correlation with surgical resectability was then determined.
Results
A total of 19 patients were identified. Overall resectability was 57.8% (n=11). Patients with Blumgart-Jarnagin stage T1 had the highest rates of resectability at 80%; patients with stage T2 and T3 disease had resectability rates of 25% and 40% respectively. Median overall survival was 13.6 months.
Cholangiocarcinomas are the second most common hepatobiliary malignancy after hepatocellular carcinoma.1 Hilar cholangiocarcinomas (HCCA), defined as tumors involving the biliary confluence, are found in the most common location of this tumor, the liver hilum.2 Currently, radical surgery offers the only chance of long-term survival because the tumor has poor response to chemotherapy and radiation therapy; however, achieving R0 resection during radical surgery is a challenge due to the complexity of the hilar anatomy. Results at expert centers only yield 5-year survival rates between 20 and 50%34567 because achieving radical resection is not always possible, and figures in the literature vary from 14 to 95%89101112 owing to the heterogeneity of existing studies.
Therefore, it is of key interest to surgeons to be able to select patients who have a high potential for surgery to be curative based on preoperative imaging. Existing staging systems for HCCA, however, such as the American Joint Committee on Cancer tumor node metastasis (AJCC TNM)13 and the Bismuth-Corlette14 systems, may be inadequate in predicting resectability.1516
In view of the limitations of existing systems, Blumgart and Jarnagin described a preoperative staging system that incorporated the effect of local tumor extent and its impact on adjacent structures317 based on non-invasive preoperative imaging and demonstrated that their system correlated better with actual surgical resectability; over the years, refinement of their system has also yielded greater accuracy in predicting resectability.18 However, the Blumgart-Jarnagin system does not include hepatic artery involvement, which generally precludes curative resection given its associated high mortality and morbidity; operative mortality has been reported to be as high as 33%.19
The primary objective of our study was to describe the correlation between preoperative Blumgart-Jarnagin staging and its correlation with surgical resectability of HCCA in our experience. The secondary objectives were to describe the utility of Blumgart and Jarnagin staging in prognosticating survival and to determine the prevalence of preoperatively, radiologically determined hepatic artery involvement in relation to actual intraoperative findings.
We identified patients who underwent surgical resection for hilar cholangiocarcinoma at Singapore General Hospital between the period of January 1, 2002, and January 1, 2013, from a prospectively maintained institutional database. We did obtain institutional review board approval.
We suspected the diagnosis of cholangiocarcinoma after preoperative radiologic imaging by either contrast-enhanced, triphasic protocol computer tomographic (CT) imaging of the abdomen and pelvis or magnetic resonance cholangiopancreatography (MRCP). For this study, we included only patients with disease in the liver hilum, as defined by tumor arising at the biliary confluence or near (<1 cm) the junction of the left and right hepatic ducts; all patients had preoperative percutaneous biliary decompression prior to surgery, and we determined future liver remnant in all patients using CT volumetry. We excluded from the study patients with preoperatively confirmed distant metastasis on imaging, cholangiocarcinoma that was not hilar in location, a histological diagnosis not consistent with cholangiocarcinoma, and patients with incomplete radiological imaging. In addition, we attempted to determine hepatic artery involvement for all patients. All cases were reviewed by a department multi-disciplinary tumor board, and all images were reviewed by a hepatopancreatobiliary radiologist.
We collected all demographic, radiological, and perioperative data using a prospectively maintained electronic clinical database (Sunrise Clinical Manager, version 5.8, Eclipsys Corporation, Atlanta, Georgia) and verified these along with supplementing with additional chart and electronic medical record review when necessary. We defined overall survival (OS) as duration from index surgery till patient demise or date of last follow-up. We graded postoperative morbidity using the Clavien-Dindo classification,2021 with major morbidity defined as Clavien-Dindo score ≥3.
All patients considered for curative resection underwent a laparotomy with full exploration of the abdominal cavity for assessment of disease burden and resectability of disease. Patients who were deemed resectable without distant metastasis proceeded with curative resection as deemed appropriate by the principle surgeon. Patients who had unresectable disease underwent a trial dissection with intraoperative biopsy of metastatic disease. When deemed necessary, a palliative bypass was constructed.
We identified a total of 43 patients between January 1, 2002, and January 1, 2013, and excluded 13 patients whose disease was equivocal for a hilar location; we also excluded 9 patients with missing or incomplete radiologic imaging. Of the remaining 21 patients, we excluded an additional 3 for histology that was not concordant with cholangiocarcinoma, giving a total of 19 patients included in the final analysis (see Fig. 2). There were 11 males (57.9%) and 8 females (42.1%), the median age was 56 years (interquartile range [IQR]: 51–65), and the median follow-up period was 406 days (IQR: 169–478 days).
Of the 19 patients considered for potentially curative resection (Table 1), 11 had resectable disease (57.8%), and for 9 (81.8%), we achieved R0 resection. Following the Blumgart-Jarnagin system, the numbers of patients with T1, T2, and T3 disease were 10 (52.6%), 4 (21.1%), and 5 (26.3%), respectively. The resectability rates for patients with Blumgart-Jarnagin T1, T2, and T3 disease were 80% (n=8), 25% (n=1), and 40% (n=2).
Of the 3 (60%) patients with unresectable Blumgart-Jarnagin T3 disease, we detected no hepatic artery involvement on preoperative imaging. Two (40%) were noted to have intra-abdominal metastatic disease and were hence precluded from curative surgery, and 1 was noted during trial dissection to have tumor involving both the common hepatic artery and portal vein and similarly did not proceed with curative surgery. One patient with T3 disease with common hepatic artery involvement underwent curative resection with a saphenous vein interposition graft and was the only one (20%) with pathologically confirmed lymph node metastasis.
In patients with Blumgart-Jarnagin T2 disease, 3 (75%) were unresectable, and preoperative imaging detected hepatic artery involvement in 1 patient who was also precluded from surgery due to multifocal liver involvement of tumor. With the remaining 2 patients, 1 was precluded due to findings of tumor encasement of the right hepatic artery while the other had significant celiac and peri-pancreatic lymphadenopathy with duodenal invasion, both of which we identified intraoperatively.
Among patients with Blumgart-Jarnagin T1 disease, we deemed 2 (20%) to have unresectable disease; we abandoned the procedure for 1 because the tumor involved a duct of the remnant non-atrophic lobe, and the other was noted intraoperatively to have extensive nodal involvement that encased both the common and right hepatic artery (Table 2); this was not noted on preoperative imaging. Of the 10 patients who had Blumgart-Jarnagin T1 disease, 1 had preoperatively identified right hepatic artery involvement but subsequently underwent curative extended right hepatectomy.
Median OS overall was 13.6 months (IQR: 5.9–25.4), and median OS for stages T1, T2, and T3 disease were, respectively, 18.5 (IQR: 7.5–29.6), 11.3 (IQR: 6.5–22.8), and 5.6 (IQR: 0.4–20.0) months. In this series, overall 30-day mortality rate was 5% (n=1), and postoperative major morbidity rate was 31.6% (n=6).
Both Bismuth-Corlette and AJCC TNM are commonly used to stage HCCA. The former considers the extent of tumor involvement of the biliary duct and was designed to guide surgical treatment,22 whereas the latter considers mainly histopathologic variables with the intention of predicting survival,23 However, the key to achieving long-term survival rests on achieving R0 resection,2425262728 and thus, there was a need for a staging system that could guide surgeons on surgical resectability. The Blumgart-Jarnagin system served to mitigate shortcomings by providing surgeons with alternative, pragmatic staging based on preoperative radiological data. Developed from a single-center cohort of 225 patients, it not only was shown to predict tumor resectability and probability of R0 resection but also correlated with survival.17 We therefore sought to study the utility of Blumgart-Jarnagin staging and its correlation with surgical resectability of HCCA in our experience.29
Patients with Blumgart-Jarnagin stage T1 in our series had the highest rate of resectability at 80%; this was not surprising given that based on the criteria, these patients had no tumor factors that generally precluded a formal resection. This is consistent with the high resectability rate (59%) in the original study by Jarnagin et al.17 and in a larger follow-up series by Matsuo et al.18 (64.3%); among those with T2 and T3 disease, resectability rates were reported to be 41.3% and 1.3%, respectively. Interestingly, there was discrepancy between patients in our series with T2 and T3 disease, with a resectability rate among those with stage T3 as opposed to T2 (40% vs. 25%). These findings were not concordant with other published ser ies,171830 most likely owing to the small numbers in our series. Nonetheless, there is the suggestion that earlier Blumgart-Jarnagin stages confer higher resectability rates than do later stages.
The utility of Blumgart-Jarnagin staging for prognosticating survival is inconclusive: The Memorial Sloan Kettering Cancer Center study18 identified a significant survival difference between T1 and T3 tumors (22.8 vs. 10.8 months) and between T2 and T3 tumors (23.0 vs. 10.8 months) but not for T1 and T2 tumors (22.8 vs. 23.0 months); the authors of that study attributed the lack of survival difference to achievement of more curative resections in the T1 and T2 groups. Although our series reflected a high rate of curative resections in the T1 group, the numbers of resections in the T2 and T3 groups differed, with a higher rate in the T3 group. Regardless, median survival rates were lower with increasing Blumgart-Jarnagin stages, with the median OS for stages T1, T2, and T3 being 18.3, 11.5 and 5.6 months, respectively. One reason for this is possibly the higher rates of metastatic disease in patients with higher Blumgart-Jarnagin stages; we identified intra-abdominal metastasis in 40% of patients with T3 disease, whereas, respectively, only 25% and 0% of patients with stages T2 and T1 disease were noted to have intraoperatively determined intra-abdominal metastasis.
Hilar cholangiocarcinoma has a tendency for longitudinal growth along bile ducts as well as radial growth that involves adjacent structures such as the hepatic artery and the portal vein.31 Although the role of radical en bloc resection with concomitant liver resection has been shown to be superior in terms of oncologic outcomes as opposed to local resection and hepaticojejunostomy,3233 the role of vascular resection remains controversial. A meta-analysis by Wu et al.34 concluded that between patients who had combined portal vein resection (PVR) and those who did not, 5-year survival rates, morbidity, and proportions of R0 resections did not differ between the 2 groups. In another meta-analysis and systematic review, PVR was associated with poorer overall survival and a lower curative resection rate than the OS and curative resection rate among patients without PVR; postoperative mortality and morbidity, however, did not differ.35 Regardless, despite poorer survival given the advanced disease stage, there is no difference in perioperative outcomes, and PVR is still performed for disease clearance in order to achieve R0 resection.
In contrast, hepatic artery resection and reconstruction was generally associated with higher morbidity and mortality without any additional survival advantage,192836 which highlights the importance of preoperative identification of vascular involvement and allows surgeons to identify patients who are candidates for potentially curative resection. In our series, a total of 6 patients had hepatic artery involvement (31.6%), with only 2 (33.3%) cases that were detected on preoperative imaging, both contrast-enhanced CT and MRCP/MRI (magnetic resonance imaging). In one systematic review and meta-analysis, the estimated sensitivity and specificity for CT in evaluating hepatic artery involvement were 84% and 93%, respectively.37 In contrast, the role of MRI/MRCP in detecting hepatic artery involvement is far less studied; sensitivities of between 58 and 73% with specificity of 93% have been reported.38
Introduction of the Blumgart-Jarnagin staging system provided surgeons with an invaluable tool for evaluating tumor resectability preoperatively; however, as the definition of unresectability evolves, so should staging systems by incorporating valuable preoperative information for guiding surgeons on disease resectability. One of the limitations with the Blumgart-Jarnagin system is the fact that it was designed on the basis of criteria for unresectability from a single institution and may not conform to the resectability criteria in other centers2939; in addition, the system does not evaluate hepatic artery or lymph node involvement or distant metastasis, which are important in determining tumor resectability. Deoliveira et al.29 described a comprehensive classification system aimed at standardizing the reporting of HCCA that incorporated features of existing staging systems and in addition evaluated extent of liver disease and volume of liver remnant as well as extent of portal vein and hepatic artery involvement, which are valuable factors in excluding patients who undergo exploration for curative intent. However, the Deoliveira system is largely descriptive as opposed to fulfilling the role of a “staging” system in that it does not correspond to severity of disease40; in addition, the system is tedious to use and requires largely intraoperative and postoperative pathologic findings to complete staging. Future work remains to develop a preoperative staging system that incorporates the extent of vascular involvement and that is sufficiently robust to predict surgical resectability.
This is a retrospective, single-center description of using the Blumgart-Jarnagin staging system to evaluate patients with HCCA, and hence, several limitations need to be highlighted. The retrospective nature of this study exposes it to confounders and bias, and furthermore, the small sample size compounds the difficulty in interpreting the findings and in meaningful analysis; however, we attempted to homogenize the study group by excluding patients with cholangiocarcinoma locations that were equivocal in the perihilar region. Other limitations included the choices of preoperative imaging; there was no preoperative standardization with regard to the imaging modality used: Specifically, we used either contrast-enhanced CT scans or contrast-enhanced MRI to evaluate disease. However, in order to mitigate the aforementioned issues, all preoperative images were read and reported by a dedicated hepatopancreatic-biliary radiologist.
In conclusion, the Blumgart-Jarnagin staging system is useful in predicting tumor resectability for HCCA. Future work includes prospective validation on a larger cohort and developing novel preoperative staging systems for predicting disease resectability.
Figures and Tables
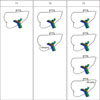 | Fig. 1Blumgart-Jarnargin staging system. T1 disease refers to tumor involvement of the biliary confluence with unilateral extension to second-order biliary radicles; T2 disease refers to tumor involving the biliary confluence with unilateral extension to second-order biliary radicles and ipsilateral portal vein involvement or ipsilateral hepatic atrophy. T3 disease refers to tumor involving the biliary confluence with bilateral extension to second-order biliary radicles or unilateral extension to second-order biliary radicles with contralateral portal vein involvement or unilateral extension to second-order biliary radicles with contralateral hepatic lobar atrophy or main/bilateral portal vein involvement. |
References
1. de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999; 341:1368–1378.

2. Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996; 224:463–473.
3. Jarnagin WR, Fong Y, Blumgart LH. The current management of hilar cholangiocarcinoma. Adv Surg. 1999; 33:345–373.
4. Launois B, Reding R, Lebeau G, Buard JL. Surgery for hilar cholangiocarcinoma: French experience in a collective survey of 552 extrahepatic bile duct cancers. J Hepatobiliary Pancreat Surg. 2000; 7:128–134.

5. Nagino M, Nimura Y, Kamiya J, Kanai M, Uesaka K, Hayakawa N, et al. Segmental liver resections for hilar cholangiocarcinoma. Hepatogastroenterology. 1998; 45:7–13.
6. Rea DJ, Munoz-Juarez M, Farnell MB, Donohue JH, Que FG, Crownhart B, et al. Major hepatic resection for hilar cholangiocarcinoma: analysis of 46 patients. Arch Surg. 2004; 139:514–523.
7. Tsao JI, Nimura Y, Kamiya J, Hayakawa N, Kondo S, Nagino M, et al. Management of hilar cholangiocarcinoma: comparison of an American and a Japanese experience. Ann Surg. 2000; 232:166–174.
8. Capussotti L, Muratore A, Polastri R, Ferrero A, Massucco P. Liver resection for hilar cholangiocarcinoma: in-hospital mortality and longterm survival. J Am Coll Surg. 2002; 195:641–647.

9. Dinant S, Gerhards MF, Rauws EA, Busch OR, Gouma DJ, van Gulik TM. Improved outcome of resection of hilar cholangiocarcinoma (Klatskin tumor). Ann Surg Oncol. 2006; 13:872–880.

10. Hemming AW, Reed AI, Fujita S, Foley DP, Howard RJ. Surgical management of hilar cholangiocarcinoma. Ann Surg. 2005; 241:693–699.

11. Hidalgo E, Asthana S, Nishio H, Wyatt J, Toogood GJ, Prasad KR, et al. Surgery for hilar cholangiocarcinoma: the Leeds experience. Eur J Surg Oncol. 2008; 34:787–794.

12. Seyama Y, Kubota K, Sano K, Noie T, Takayama T, Kosuge T, et al. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg. 2003; 238:73–83.

13. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–1474.

14. Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992; 215:31–38.

15. Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011; 8:512–522.

16. Zervos EE, Osborne D, Goldin SB, Villadolid DV, Thometz DP, Durkin A, et al. Stage does not predict survival after resection of hilar cholangiocarcinomas promoting an aggressive operative approach. Am J Surg. 2005; 190:810–815.

17. Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BS J, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001; 234:507–517.

18. Matsuo K, Rocha FG, Ito K, D'Angelica MI, Allen PJ, Fong Y, et al. The Blumgart preoperative staging system for hilar cholangiocarcinoma: analysis of resectability and outcomes in 380 patients. J Am Coll Surg. 2012; 215:343–355.

19. Miyazaki M, Kato A, Ito H, Kimura F, Shimizu H, Ohtsuka M, et al. Combined vascular resection in operative resection for hilar cholangiocarcinoma: does it work or not? Surgery. 2007; 141:581–588.

20. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009; 250:187–196.
21. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240:205–213.
22. Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975; 140:170–178.
23. Benson AB 3rd, D'Angelica MI, Abbott DE, Abrams TA, Alberts SR, Saenz DA, et al. NCCN Guidelines Insights: Hepatobiliary Cancers, Version 1.2017. J Natl Compr Canc Netw. 2017; 15:563–573.

24. Cannon RM, Brock G, Buell JF. Surgical resection for hilar cholangiocarcinoma: experience improves resectability. HPB (Oxford). 2012; 14:142–149.

25. Cho MS, Kim SH, Park SW, Lim JH, Choi GH, Park JS, et al. Surgical outcomes and predicting factors of curative resection in patients with hilar cholangiocarcinoma: 10-year single-institution experience. J Gastrointest Surg. 2012; 16:1672–1679.

26. Nuzzo G, Giuliante F, Ardito F, Giovannini I, Aldrighetti L, Belli G, et al. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg. 2012; 147:26–34.
27. Rocha FG, Matsuo K, Blumgart LH, Jarnagin WR. Hilar cholangiocarcinoma: the Memorial Sloan-Kettering Cancer Center experience. J Hepatobiliary Pancreat Sci. 2010; 17:490–496.

28. Miyazaki M, Ito H, Nakagawa K, Ambiru S, Shimizu H, Shimizu Y, et al. Aggressive surgical approaches to hilar cholangiocarcinoma: hepatic or local resection. Surgery. 1998; 123:131–136.

29. Deoliveira ML, Schulick RD, Nimura Y, Rosen C, Gores G, Neuhaus P, et al. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology. 2011; 53:1363–1371.

30. Ding G, Yang Y, Cao L, Chen W, Wu Z, Jiang G. A modified Jarnagin-Blumgart classification better predicts survival for resectable hilar cholangiocarcinoma. World J Surg Oncol. 2015; 13:99.

31. Hayashi S, Miyazaki M, Kondo Y, Nakajima N. Invasive growth patterns of hepatic hilar ductal carcinoma. A histologic analysis of 18 surgical cases. Cancer. 1994; 73:2922–2929.

32. Fong Y, Blumgart LH, Lin E, Fortner JG, Brennan MF. Outcome of treatment for distal bile duct cancer. Br J Surg. 1996; 83:1712–1715.

33. Klempnauer J, Ridder GJ, von Wasielewski R, Werner M, Weimann A, Pichlmayr R. Resectional surgery of hilar cholangiocarcinoma: a multivariate analysis of prognostic factors. J Clin Oncol. 1997; 15:947–954.

34. Wu XS, Dong P, Gu J, Li ML, Wu WG, Lu JH, et al. Combined portal vein resection for hilar cholangiocarcinoma: a meta-analysis of comparative studies. J Gastrointest Surg. 2013; 17:1107–1115.

35. Chen W, Ke K, Chen YL. Combined portal vein resection in the treatment of hilar cholangiocarcinoma: a systematic review and meta-analysis. Eur J Surg Oncol. 2014; 40:489–495.

36. Abbas S, Sandroussi C. Systematic review and meta-analysis of the role of vascular resection in the treatment of hilar cholangiocarcinoma. HPB (Oxford). 2013; 15:492–503.

37. Ruys AT, van Beem BE, Engelbrecht MR, Bipat S, Stoker J, Van Gulik TM. Radiological staging in patients with hilar cholangiocarcinoma: a systematic review and meta-analysis. Br J Radiol. 2012; 85:1255–1262.

38. Lee MG, Park KB, Shin YM, Yoon HK, Sung KB, Kim MH, et al. Preoperative evaluation of hilar cholangiocarcinoma with contrast-enhanced three-dimensional fast imaging with steady-state precession magnetic resonance angiography: comparison with intraarterial digital subtraction angiography. World J Surg. 2003; 27:278–283.

39. Nagino M. Perihilar cholangiocarcinoma: a surgeon's viewpoint on current topics. J Gastroenterol. 2012; 47:1165–1176.

40. Nagino M. Perihilar cholangiocarcinoma: a much needed but imperfect new staging system. Nat Rev Gastroenterol Hepatol. 2011; 8:252–253.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download