Abstract
Backgrounds/Aims
A wide range of surgical approaches has been described for hepatic hydatidosis aiming primarily at the reduction of disease recurrence and minimization of postoperative morbidity.
Methods
A database analysis of patients with liver hydatidosis who underwent different surgical procedures between March 2010 and May 2016 was performed.
Results
A total of 21 patients with cystic echinococcosis (CE) and four cases of alveolar echinococcosis (AED) were detected. Nine patients manifested recurrent disease at presentation. Among CE cases, 5 underwent partial cystectomy (2 laparoscopic and 3 open), 9 cysto-pericystectomy (7 open and 2 robotic) and 7 hepatectomies (1 central, 4 right, 1 left and 1 right trisectionectomy). Living donor liver transplantation was performed in 3 patients with AED and the fourth patient underwent right trisectionectomy with en bloc resection of hepatic flexure and right diaphragm. Seven developed Clavien grade II and three grade III complications. The mean follow-up of CE was 34.19±19.75 months. One patient of laparoscopic partial cystectomy developed disease recurrence at 14 months. No recurrence was detected at a mean follow-up of 34±4.58 months following LDLT and at 24 months following multivisceral resection for AED.
Hydatidosis is a zoonotic disease caused by Echinococcus species (E.granulosus, multilocularis, and oligarthus). Cystic echinococcosis (CE) is caused by E. granulosus and alveolar echinococcal disease (AED) by E. multilocularis. Both the CE and AED involve mainly liver, constituting approximately 75% and 90% of the disease burden, respectively.12
CE largely remains asymptomatic and is usually detected incidentally. Symptoms appear mainly because of the larger lesion size or its complications (rupture/suppuration).3 AED also remains indolent and asymptomatic during the incubation period of 5-15 years. AED also masquerades as malignancy due to its infiltrative nature. The clinical presentation may be attributed to its infiltrative nature, manifesting as painful abdomen (30%) or cholestatic jaundice (30%). However, one-third of all cases are detected incidentally.4
Imaging by ultrasonography (USG) and contrast-enhanced computed tomography (CECT) facilitates diagnosis, staging and classification, and surgical planning of both CE and AED.56
The treatment of liver hydatids ranges from medical management to liver transplantation in AED. Medical management is associated with a high degree of failure and disease recurrence (20–40%).7 Similarly, the reported incidence of recurrence and morbidity following conservative surgery in CE is 3–30% and 21%–80%, respectively.78 Radical surgery increases the operative risk but lowers the likelihood of recurrence in CE. Advances in hepatic resection over the last few decades have increased the safety of major hepatic resection. Currently, hepatic resection remains the mainstay of treatment with a high success rate in CE.91011 Similarly, in the absence of adequate surgical treatment, mortality is close to 90% in AED.4 Surgical resection is the only optimal treatment available for AED because of its infiltrative nature and metastatic potential.12 Surgical resections include multi-visceral resection and liver transplantation accompanied with or without visceral and vascular resection and reconstruction.
In this study, we report the spectrum of surgical interventions used to manage liver hydatid disease and their outcomes.
This study is a retrospective review of a prospectively maintained database of patients with hydatid liver disease (CE and AED) presented or referred to our institute from March 2010 to May 2016. The study has been approved by the institutional review board of our institution (MICR-766/2017) and conducted in accordance with the Declaration of Helsinki. All patients diagnosed with AED were referred to our center from central Asian countries. Our diagnostic workup for the study consisted of complete blood counts, liver function tests, and hydatid serology with heme-agglutination tests, and imaging studies comprising USG, contrast-enhanced computed tomography (CECT) and/or dynamic magnetic resonance imaging (MRI).
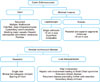
Surgical approach algorithms for CE and AED are depicted in Fig. 1.
In general, complete surgical resection was the preferred treatment option at our institution. Four types of surgical approaches were followed in CE in the order of preference. Anatomical resection was usually performed for a recurrent and complicated cyst or multiple cysts localized to one lobe of the liver provided that the future liver remnant was adequate.
Cysto-pericystectomy (Closed or Open) was usually performed for solitary large cysts with high suspicion of cystobiliary communication and inadequate future liver remnant. When the cyst was initially too bulky to allow for closed cysto-pericystectomy, the cyst was aspirated and drained using a large-bore suction aspirator (open cysto-pericystectomy). The cyst cavity was then filled with a scolicidal agent (10% povidone-iodine). The liver was isolated with laparotomy pads soaked in the scolicidal agent, followed by suture closure with polypropylene to avoid spillage. The same method as in open surgery was adopted during robotic cysto-pericystectomy. Biliary leaks were tested with methylene blue injection and intraoperative cholangiogram. All demonstrated biliary leaks were ligated with 5-0 polydioxanone sutures. Diaphragmatic resection and repair (primary or mesh patch) was carried out for invasive cysts or cysts adhering to the diaphragm.
Partial cystectomy was performed when the cyst wall abutted the portal vein (peri-hilar) or major hepatic vein (due to inflammatory adhesions, deemed unsafe for complete cystectomy) or the cyst interfered with the major hepatic vein-inferior vena cava junction superiorly. Partial cystectomy was also performed when anatomical resection was contraindicated due to the low future liver remnant. The cyst cavity was left open after excision of the cyst wall. The cyst wall adherent or close to vessels was left behind and fulgurated. Percutaneous or surgical (open/laparoscopic) drainage is indicated for infected cyst and abscess. None of the patients underwent this procedure in the present case series.
Minimally invasive approach (laparoscopy/robotic) was adapted according to patient preference and procedural feasibility. Laparoscopic cysto-pericystectomy, although feasible, was not performed in view of the perceived difficulty involving intracorporeal suturing in a deep cavity.
The surgical approaches against AED entailed either resection or liver transplantation. Resection including multivisceral resection was preferred in view of the diffuse nature of the echinococcal disease. Similarly, living donor liver transplantation (LDLT) was performed in patients with diffuse bilobar echinococcal disease, encasing hilum or recurrence post resection precluding further safe resection. Other visceral and vascular involvement was not a contraindication to LDLT provided that they could be safely excised and reconstructed. The prevailing organ donation law in India prevents listing AED patients for deceased donor organ donation as they were referred from various other central Asian countries.
Preoperative or postoperative antiparasitic agents were routinely administered. Most of our patients had already received albendazole for variable duration before approaching us. Patients not treated with albendazole were started at a dose of 15 mg/kg in two divided doses at least 7 days prior to surgery and continued for 3 to 6 months post surgery for CE and 2 years for AED (as per recommendations by WHO).4
Post LDLT, all recipients received triple immunosuppressant therapy as per institutional protocol (tacrolimus/mycophenolate mofetil/steroid). Steroids were tapered and stopped at 3 months followed by mycophenolate mofetil at 1 year in all three recipients. All postoperative complications were recorded and graded according to the Clavien-Dindo classification.13
The patients were followed up after surgery at monthly intervals for 3 months, and later every 3 to 6 months for 3 years, and then annually. At follow-up, routine blood investigations including hydatid serology (when recurrence was suspected) and imaging by USG or CT were conducted. Disease recurrence was defined on the basis of evidence of 1 or more novel hydatid cysts on imaging.
Data were presented as mean±standard deviation (SD) for continuous variables. Continuous variables were analyzed using independent Student's t-test. A two-tailed p-value of <0.05 was considered statistically significant. All statistical data were generated using SPSS 20 (Chicago, IL, USA).
A total of 25 patients (including 13 males and 12 females) of hepatic hydatidosis underwent different surgical procedures during the study period at our institute. The clinical profile and details of surgical approaches are shown in Table 1. The mean age of study cohort was 30.96±15.11 years. Twenty-one patients underwent CE and four patients had AED. Nine patients presented with recurrent disease following various procedures, which were performed elsewhere [percutaneous aspiration injection and re-aspirtation (PAIR) in 2, laparoscopic partial cystectomy (n=2), pericystectomy (n=3), right hepatectomy (n=1) and segment VIII resection (n=1)]. The most common presentation involved nonspecific and vague abdominal discomfort in 84% of the patients. Two patients presented with recurrent cholangitis due to cystobiliary communication and both were diagnosed with preoperative endoscopic retrograde cholangiopancreatography (ERCP) to rule out intraductal cysts. Both were de novo cysts. At presentation, 19 patients had positive hydatid serology. Multiple cysts were present in 10 patients with CE, with the cyst size ranging from 5 cm to 20 cm.
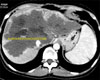
Out of 21 patients with CE, 5 patients underwent partial cystectomy (2 laparoscopic and 3 open). Both the patients who underwent laparoscopic partial cystectomy had a superficial lesion. The first patient carried a 4 cm×5 cm×4 cm-sized lesion involving the segments VI and VII, whereas the second patient harbored a predominantly right-sided lesion extending to the segment IV (size: 4 cm×4.5 cm×4 cm). Partial cystectomy was performed in open cases: in the first case, the cyst wall abutted the right hepatic vein and in the other two cases middle hepatic vein. As the dissection between cyst wall and veins was considered to be unsafe, the content was evacuated, and part of the cyst wall adherent to major vein was left behind and fulgurated. Two patients underwent robotic cysto-pericystectomy. Both the patients carried a lesion located predominantly posterior. The first case carried a 5 cm×5 cm×4 cm-sized lesion in the segment VII and in the second case 8 cm×7 cm×8 cm-sized lesion in the segments VI and VII extending to the segment VIII (Fig. 2). One patient underwent open central hepatectomy, 4 right hepatectomy, one right trisectionectomy and one left hepatectomy. Two patients in the right hepatectomy group underwent preoperative CECT scan suggestive of cystobiliary communication.
The patient with recurrent CE developed significantly greater blood loss (650±536.6 vs 241.6±146.3, p=0.01) and increased operative duration (418±168 vs 270.7±147.9, p=0.04) compared with de novo CE. However, the length of hospital stay after surgery was similar between the groups (9.1±5.4 vs 7.4±3, p=0.36).
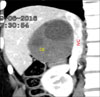
All the 4 patients were P4N1M0 according to PNM classification for alveolar echinococcosis. Three patients underwent LDLT including one with disease recurrence post right hepatectomy in the remnant lobe involving left portal vein and two patients manifested diffuse hydatidosis of the entire liver involving both inflow and outflow vessels (Fig. 3). The mean operative duration and intraoperative blood loss for patients undergoing LDLT was 636.7±20.8 minutes and 1400±346.4 mL, respectively. All the patients underwent right lobe graft with a graft-recipient weight ratio (GRWR) of >1 in two patients and 0.81 in one patient. Two patients required vena cava replacement. End-to-end porto-portal anastomosis (recipient main portal vein to the graft right portal vein interposition extension graft was required in two patients), and arterio-arterial anastomoses (recipient right hepatic artery to graft right hepatic artery) were performed. Duct-to-duct biliary anastomosis was performed in all three cases. The mean hospital stay was 11.3±1.2 days.
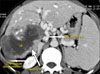
The fourth patient had a mass involving right lobe extending to segment 4, involving right hepatic artery, diaphragm, and colon (Fig. 4). The liver remnant was adequate (40%) and resection was deemed feasible and safe. Right trisectionectomy, segmental colectomy, partial right diaphragmatic excision with Rouxen Y hepaticojejunostomy, and mesh repair of the diaphragm were performed.
On the Clavien-Dindo scale, 3 patients had grade I, 7 patients had grade II, and 3 patients had grade III complications (Table 2). Out of 3 patients with Grade III complication, 2 developed a bile leak, whereas one patient required percutaneous drainage of the pelvic collection. Both patients with bile leak had cut surface leak, recovery was expedited by ERCP and stenting. The stent was removed after 6 weeks. Out of the two patients with bile leak, one underwent left hepatectomy (multiple lesions in left lobe) and other had right hepatectomy for multiple lesions involving segments 5, 7 and 8 (largest lesion measuring 9 cm×7 cm×7 cm).
The mean follow-up was 34.2±19.8 months (range: 6–66 months) for CE. A single case of recurrence following laparoscopic partial cystectomy for a lesion in right lobe (4 cm×4.5 cm×4 cm) was detected at 14 months and resolved via right hepatectomy.
Currently, all three LDLT patients are on single immunosuppressant (tacrolimus) therapy maintaining good graft function. No evidence of radiological recurrence was detected at a mean follow-up of 34±4.6 months following LDLT and at 24 months follow-up following multivisceral resection for AED. No donor morbidity or mortality occurred and all are on regular follow-up.
This study addresses the surgical management of hepatic hydatidosis at a tertiary care center, where the maximum disease burden is in the form of recurrent or complicated disease. Thirty-six percent of our patients had recurrent disease.
Medical management alone in CE yielded a success rate of only 10%–30%, failure and recurrence rates of 20%–40% and 3%–30%,7814 respectively.
Surgical excision of cysts can be curative for CE. Despite the availability of surgical procedures controversy about the most optimal procedure still remains.31516 Conservative procedures are safe and less complex than radical surgery, however, the advantage is offset by postoperative complications of 6–47%.1117 Non-comparative retrospective studies established the safety and effectiveness of radical surgery. A comparative retrospective study by Aydin et al.17 of 242 patients, reported significantly higher morbidity and recurrence rates with conservative surgery (11% vs. 3%; 24% vs. 3%). Similar results were shown by Tagliacozzo et al.18 in the study that included 454 patients. Yuksel et al.19 have compared radical surgery versus conservative surgery. In this randomized study comprising 32 patients, radical surgery scored over conservative surgery both in terms of recurrence (p=0.04) and complications (p=0.01). The overall recurrence rates following radical surgery range from 0% to 4.65% in contrast to 4.65% to 25% following conservative approach.2021
The choice of surgical technique and the radical procedure involved is influenced by size, site, type of the cyst, complications, relation to bile ducts and blood vessels, clinical presentation and surgical expertise.910 In general, surgical excision was the preferred treatment option at our institution. Conservative surgery was considered when the entire burden of hepatic cysts could not be safely excised.
In our series, 76% of CE patients underwent radical surgery. History of previous surgery and presence of adhesions increases the complexity of the surgery. We found that patients who underwent recurrent CE manifested significantly greater intraoperative blood loss and endured longer operation compared with de-novo CE. No mortality was seen, and the overall morbidity was 52%. However, Clavien-Dindo grade 3 or higher complications were 12%, which is comparable to the current rates reported in the literature.7,19
The use of a minimally invasive approach for managing liver hydatid cysts was first documented in 1992.22 The indications have expanded through the years. The only excluding criteria for minimally invasive intervention are deep intra-parenchymal cysts, posterior cysts situated close to the vena cava, more than 5 cysts, multilocular cysts with thick and calcified walls.23 According to our institutional protocol, laparoscopy was offered to patients diagnosed with single superficial lesions measuring less than 5 cm, without suspicion of cysto-biliary communication. Patients with superior and posterior cyst location with an exophytic component were considered for robotic intervention. The robotic approach offers advantages of 3D visualization through the stereo endoscope, tremor reduction, motion scaling and an additional degree of freedom with wristed instrument allows safe parenchymal dissection and intracorporeal suturing for hemostasis and biliostasis, thus overcoming the limitations of laparoscopy.24 All the patients who had recurrent disease, calcified deep intra-parenchymal lesions, lesions abutting major vessels and with cystobiliary communication were considered for an open approach.
Postoperative morbidity in laparoscopic studies ranges from 8% to 25%, compared with 12% to 63% in open series.25 The short-term recurrence varies from 0% to 9% post-laparoscopy, while in open series it is even higher (0% to 30%). The favorable laparoscopic outcomes in liver hydatid disease may be related to not only the advantages of minimally invasive surgery but also the selection criteria for the patients. The advantages of a laparoscopic approach compared with open surgery include a shorter hospital stay, a lower incidence of wound infection and less postoperative pain. However, the pitfalls in the form of difficulties in accessing cysts in certain locations, an increased risk of cyst content spillage, and difficulty in aspirating cysts with viscous contents cannot be overlooked.25
No randomized study has evaluated a minimally invasive approach for the treatment of liver hydatid cyst. Most published studies are non-comparative observational studies stating the safety of laparoscopic approach with objectively low conversion rates and no mortality.26 Our series comprised 4 patients who underwent a minimally invasive approach (2 laparoscopic and 2 robotic), without any conversion. However, a single patient had recurrence following laparoscopic partial cystectomy.
AED is slow growing and detected late in the course of disease.4 It is potentially fatal with a mortality rate exceeding 90% at 10 years if left untreated. Treatment options in AED include radical surgery, interventional procedures, long-term chemotherapy, and finally liver transplantation.4 The resectability rate is only 25%–40% and generally depends on the stage of the disease, age, patient's general condition, and surgical experience.27
At our institute, the feasibility of resection was assessed in all patients and considered when single lobe, adequate remnant, and inflow to remnant were involved. LDLT was considered in a patient with total inflow involvement, diffuse bilobar disease, secondary biliary cirrhosis or recurrence of AED post resection precluding further safe resection.
The infiltrative nature of the alveolar disease, associated inflammation, late detection and involvement of surrounding structures such as diaphragm, colon, and major vessels increases the complexity of the surgery. Multivisceral resection may be indicated more often than not involving diaphragm and colon. Both recipient hepatectomy and implantation is technically more challenging. Bresson-Hadni et al.28 had reported a perioperative mortality rate as high as 26%. The dissection around the cava and porta is much more difficult compared with the usual cirrhotic patients. Many cases warrant venacaval replacement. The hilar structure may be too short and require portal vein, and hepatic artery extension via interposition grafts, and bilioenteric anastomosis for biliary reconstruction.29 In our series, two patients required IVC replacement and portal vein extension grafts. However, no need for such extension was observed in arterial reconstruction and all three patients underwent duct-to-duct biliary anastomoses. Liver transplantation has been performed in AED since the 1980s and the outcome has been good with 5-year actuarial survival close to 70% and recurrence-free survival of 58%.30 All three patients in our series are surviving with good graft function at a follow-up of 34±4.6 months.
The main limitation of our study relates to the fewer patients investigated. However, the study goal was to highlight disease management in the present era, with too many paradigm shifts pertaining to the safety of liver resections, parenchymal preservation, feasibility and applicability of minimal access surgery.
In summary, hepatic hydatidosis is a disease with variable presentation requiring tailored surgical approaches, for improved effectiveness, safety, and minimization of recurrences. The radical surgical procedure is a safe and effective option for CE, especially for complicated and recurrent disease. The role of conservative surgery should not be undermined considering the benign nature of the disease (CE) and the patient's safety profile. AED warrants complete surgical excision as the first option even if it entails multi-visceral or vascular resections and liver transplantation when excision is contraindicated.
Figures and Tables
 | Fig. 3Computed tomography showing diffuse bilobar involvement of alveolar echinococcosis in a patient who underwent right lobe living donor liver transplantation. |
ACKNOWLEDGEMENTS
The authors acknowledge Shivani Sharma, research coordinator, for assistance with the database.
References
1. Grosso G, Gruttadauria S, Biondi A, Marventano S, Mistretta A. Worldwide epidemiology of liver hydatidosis including the mediterranean area. World J Gastroenterol. 2012; 18:1425–1437.

3. Menezes da Silva A. Hydatid cyst of the liver-criteria for the selection of appropriate treatment. Acta Trop. 2003; 85:237–242.

4. Brunetti E, Kern P, Vuitton DA. Writing Panel for the WHOIWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010; 114:1–16.

5. Graeter T, Kratzer W, Oeztuerk S, Haenle MM, Mason RA, Hillenbrand A, et al. Proposal of a computed tomography classification for hepatic alveolar echinococcosis. World J Gastroenterol. 2016; 22:3621–3631.

6. WHO Informal Working Group. International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop. 2003; 85:253–261.
7. Gomez I, Gavara C, López-Andújar R, Belda Ibáñez T, Ramia Ángel JM, Moya Herraiz Á, Orbis Castellanos F, et al. Review of the treatment of liver hydatid cysts. World J Gastroenterol. 2015; 21:124–131.
8. Prousalidis J, Kosmidis CH, Fahantidis E, Harlaftis N, Aletras O. Surgical treatment of multiple cystic echinococcosis. HPB (Oxford). 2004; 6:110–114.

9. Akbulut S, Senol A, Sezgin A, Cakabay B, Dursun M, Satici O. Radical vs conservative surgery for hydatid liver cysts: experience from single center. World J Gastroenterol. 2010; 16:953–959.
10. Bresson-Hadni S, Koch S, Miguet JP, Gillet M, Mantion GA, Heyd B, et al. European group of clinicians. indications and results of liver transplantation for echinococcus alveolar infection: an overview. Langenbecks Arch Surg. 2003; 388:231–238.
11. Alfieri S, Doglietto GB, Pacelli F, Costamagna G, Carriero C, Mutignani M, et al. Radical surgery for liver hydatid disease: a study of 89 consecutive patients. Hepatogastroenterology. 1997; 44:496–500.
12. Torgerson PR, Schweiger A, Deplazes P, Pohar M, Reichen J, Ammann RW, et al. Alveolar echinococcosis: from a deadly disease to a well-controlled infection. relative survival and economic analysis in Switzerland over the last 35 years. J Hepatol. 2008; 49:72–77.

13. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240:205–213.
14. Rinaldi F, Brunetti E, Neumayr A, Maestri M, Goblirsch S, Tamarozzi F. Cysticechinococcosis of the liver: A primer for hepatologists. World J Hepatol. 2014; 6:293–305.
15. Martel G, Ismail S, Bégin A, Vandenbroucke-Menu F, Lapointe R. Surgical management of symptomatic hydatid liver disease: experience from a Western centre. Can J Surg. 2014; 57:320–326.

16. Silva MA, Mirza DF, Bramhall SR, Mayer AD, McMaster P, Buckels JA. Treatment of hydatid disease of the liver. evaluation of a UK experience. Dig Surg. 2004; 21:227–233.
17. Aydin U, Yazici P, Onen Z, Ozsoy M, Zeytunlu M, Kiliç M, et al. The optimal treatment of hydatid cyst of the liver: radical surgery with a significant reduced risk of recurrence. Turk J Gastroenterol. 2008; 19:33–39.
18. Tagliacozzo S, Miccini M, Amore Bonapasta S, Gregori M, Tocchi A. Surgical treatment of hydatid disease of the liver: 25 years of experience. Am J Surg. 2011; 201:797–804.

19. Yuksel O, Akyürek N, Sahin T, Salman B, Azili C, Bostanci H. Efficacy of radical surgery in preventing early local recurrence and cavity-related complications in hydatic liver disease. J Gastrointest Surg. 2008; 12:483–489.

20. Birnbaum DJ, Hardwigsen J, Barbier L, Bouchiba N, Le Treut YP. Is hepatic resection the best treatment for hydatid cyst? J Gastrointest Surg. 2012; 16:2086–2093.

21. Vennarecci G, Manfredelli S, Guglielmo N, Laurenzi A, Goletti D, Ettorre GM. Major liver resection for recurrent hydatid cyst of the liver after suboptimal treatment. Updates Surg. 2016; 68:179–184.

22. Katkhouda N, Fabiani P, Benizri E, Mouiel J. Laser resection of a liver hydatid cyst under videolaparoscopy. Br J Surg. 1992; 79:560–561.

23. Tuxun T, Zhang JH, Zhao JM, Tai QW, Abudurexti M, Ma HZ, et al. World review of laparoscopic treatment of liver cystic echinococcosis--914 patients. Int J Infect Dis. 2014; 24:43–50.

24. Chautems R, Buhler L, Gold B, Chilcott M, Morel P, Mentha G. Long term results after complete or incomplete surgical resection of liver hydatid disease. Swiss Med Wkly. 2003; 133:258–262.
25. Zaharie F, Bartos D, Mocan L, Zaharie R, Iancu C, Tomus C. Open or laparoscopic treatment for hydatid disease of the liver? a 10-year single-institution experience. Surg Endosc. 2013; 27:2110–2116.

26. Jarufe N, Galindo JL, Bächler JP, Ahumada V, Rebolledo R, Crovari F, et al. Radical laparoscopic treatment for hepatic hydatid disease. J Gastrointest Dig Syst. 2016; 6:419.

27. Bresson-Hadni S, Vuitton DA, Bartholomot B, Heyd B, Godart D, Meyer JP, et al. A twenty-year history alveolar echinococcosis analysis of a series of 117 patients from eastern France. Eur J Gastroenterol Hepatol. 2000; 12:327–336.
28. Bresson-Hadni S, Blagosklonov O, Knapp J, Grenouillet F, Sako Y, Delabrousse E, et al. Should possible recurrence of disease contraindicate liver transplantation in patients with end-stage alveolar echinococcosis? a 20-year follow-up study. Liver Transpl. 2011; 17:855–865.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download