Abstract
Backgrounds/Aims
Hepatocellular carcinoma (HCC) recurrence remains a great concern following hepatic resection and liver transplantation. We investigated the metformin-induced cytotoxic effects on sorafenib in an in vitro study using HCC cell lines.
Methods
This research was conducted through an in vitro study using one HepG2.2.15 liver tumor and two patient-derived graft HCC cell lines.
Results
An in vitro study revealed noticeable cytotoxic effects of metformin as well as noticeable synergistic cytotoxic effects of metformin and sorafenib on cell viability. Assays for the mechanisms of action of antitumor effects revealed that alpha-fetoprotein expression was suppressed by both metformin and sorafenib, but no synergistic effect was observed. LC3-I and LC3-II assays revealed the synergistic upregulation of autophagy and assays for IL-1β, IL-6, p53, and TNF-α revealed the synergistic upregulation of cell damage and apoptosis. In contrast, metformin did not affect HBx expression, thus no noticeable synergistic effect was considered to be present.
Go to : 
Hepatocellular carcinoma (HCC) is one of the most common malignancies as well as a leading cause of cancer-related death. HCC is initially treated with various locoregional therapies including resection, but tumor recurrence or progression can result in such treatments no longer being applicable. Thus, a considerable number of patients with advanced HCC undergo systemic chemotherapy including sorafenib. Sorafenib is currently regarded to be the first-line chemotherapeutic agent for treating advanced HCC following reports of prolonged survival periods in two randomized controlled trials.12 However, the therapeutic effect of sorafenib is often suboptimal, and most patients still experience tumor progression.3 Thus, there is an essential need to discover add-on agents that can enhance the therapeutic effects of sorafenib.
Metformin is a biguanide used in the treatment of type 2 diabetes mellitus. It is capable of inhibiting cancer cell growth by inducing cell cycle arrest and enhancing apoptosis.4567 A considerable number of studies have suggested that metformin plays a chemopreventive role in other cancers, as well as its association with a reduced risk of HCC,8910 suggesting the antitumor effects of metformin. We recently reported that metformin administration was associated with reduced tumor recurrence and helped induce significant improvements in overall patient survival in patients who underwent hepatic resection for HCC.11 It has also been reported that combined metformin and sorafenib can suppress proliferation and induce autophagy of HCC in vitro and in vivo through targeting the mammalian target of rapamycin (mTOR) pathway.12
Therefore, the present study intended to assess whether the add-on use of metformin to sorafenib could induce synergistic antitumor effects on in vitro study with HCC cell lines.
Go to : 
This study was an in vitro study focused on assessing whether or not the concurrent administration of metformin and sorafenib has a synergistic cytotoxic effect on liver tumor cell lines. The study protocols were approved by the Ethical Committee of Animal Study in the Asan Institute of Life Sciences.
Three liver tumor cell lines were used: one established cell line and two patient-derived xenograft tumor cell lines. First, the HepG2.2.15 cell line (Korean Advanced Institute of Science and Technology), which is derived from the human hepatoblastoma cell line HepG2 through hepatitis B virus (HBV) transfection, was chosen based on the fact that most HCC patients in Korea have an associated HBV infection. Second, two patient-derived xenograft tumor cell lines were established. HBV-associated human HCC tissue was implanted into a non-obese diabetic/severe combined immunodeficiency mouse. The established first-generation xenograft tumor was serially implanted into severe combined immunodeficiency mice and nude mice in order to expand the xenograft tumors. This tumor was then harvested so as to establish a new patient-derived xenograft tumor cell line. Detailed procedures were as previously reported.13
The cytotoxic effects of metformin and sorafenib were evaluated using the three liver tumor cell lines mentioned above. In line with the therapeutic range in patients with type II diabetes, the in vitro drug concentrations were determined as 5–10 mmol/mL for metformin and 5–20 µmol/mL for sorafenib.14 The duration of drug administration was 20 hours.
In order to assess cell viability, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed using 12-well plates. Optical density was assessed at 550 nm using a microplate reader (Bio-Rad). Cell survival was expressed as the percentage of absorbance of drug-treated cells relative to that of untreated cells. MTT was purchased from Duchefa (Haarlem, the Netherlands). Cells were also observed under fluorescence microscopy following 4′,6-diamidino-2-phenylindole-Hoechst staining (Sigma-Aldrich; Poole, Dorset, UK).
In order to assess the synergistic antitumor effects of metformin and sorafenib, reverse transcription polymerase chain reactions (RT-PCR) of α-fetoprotein (AFP) as a marker of tumor suppression, of microtubule-associated protein light chain 3B (LC3B-I and LC3B-II) as a marker for autophagy; and interleukin-1β (IL-1β), interleukin-6 (IL-6), p53, and tumor necrosis factor-α (TNF-α) as markers for cell damage and apoptosis were performed. Details on the PCR method are available elsewhere.15
RT-PCR to measure HBV X (HBx) expression was also performed in order to assess whether or not metformin could inhibit HBV-derived HCC by inhibiting HBV replication. Western blot assay was performed in order to assess HBx protein expression and apoptosis. Cell extracts were separated by polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. After blocking, the membrane was incubated with a primary antibody against HBx and actin, followed by incubation with a secondary antibody. Antibodies against HBx (ALX-804-278-C100) were purchased from Enzo Life Sciences (Farmingdale, NY, USA) and those against actin (AC-15, A3854) were purchased from Sigma-Aldrich (Poole, Dorset, UK). Protein in the samples was detected using a Supersignal pico-enhanced chemiluminescence kit (Pierce, Rockford, IL, USA). Unless otherwise specified, cell lysates containing 20 µg of protein were analyzed.
Continuous variables are reported as mean with standard deviation or median with range, and were compared using Student's t-test. A p-value of <0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed using GraphPad Prism version 5.0 (GraphPad, La Jolla, CA, USA) and SPSS version 22 (IBM, New York, NY, USA).
Go to : 
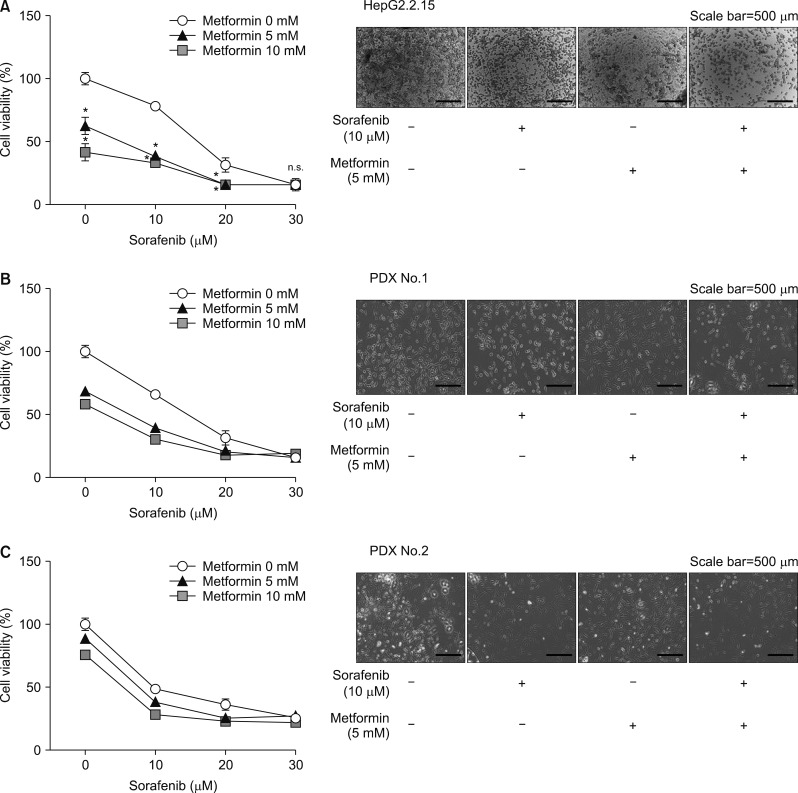
The MTT assay for cell survival assessment showed a concentration-dependent decrease in cell survival after 20-hour treatment with 5–20 µmol/mL sorafenib and 5–10 mmol/mL metformin in the HepG2.2.15 and two patient-derived xenograft cell lines. Combining the drugs led to a further concentration-dependent decrease in cell survival (Fig. 1A–C).
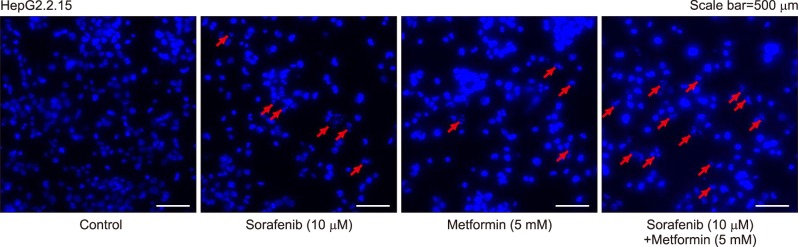
Fluorescence microscopy with 4′,6-diamidino-2-phenylindole-Hoechst staining showed noticeable apoptosis following metformin administration in all three cell lines, which was comparable to that of sorafenib administration. The highest levels of apoptosis were observed after combination therapy (Fig. 2).
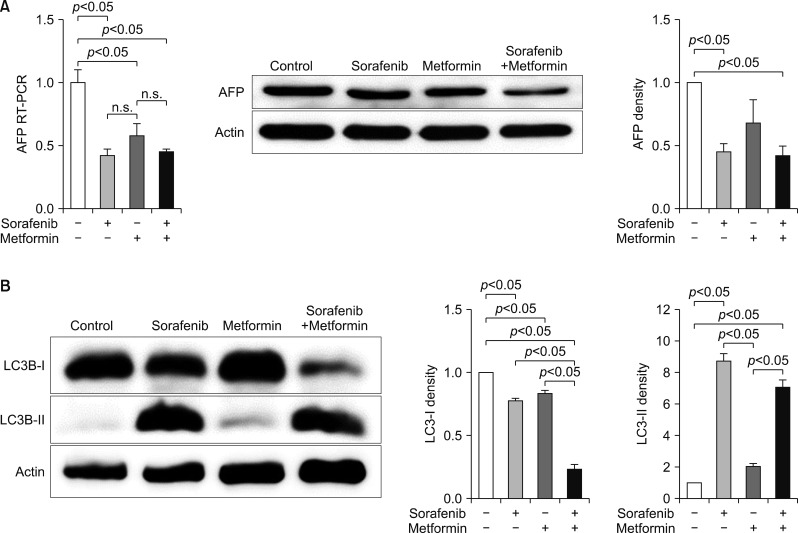
RT-PCR assays to assess the mechanisms of action of antitumor effects revealed that AFP expression was suppressed by both metformin and sorafenib, but no synergistic effect was observed (Fig. 3A). LC3-I and LC3-II assays revealed the synergistic upregulation of autophagy (Fig. 3B).
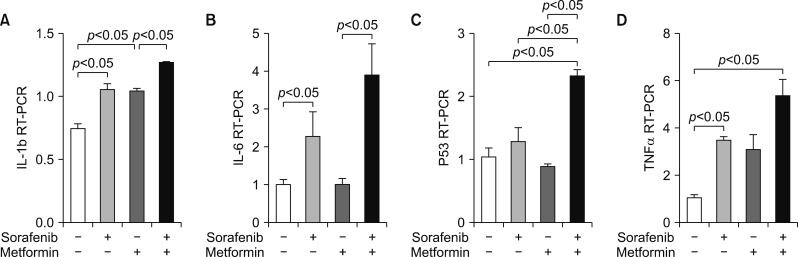
RT-PCR assays for IL-1β, IL-6, p53, and TNF-α revealed the synergistic upregulation of cell damage and apoptosis (Fig. 4C, D).
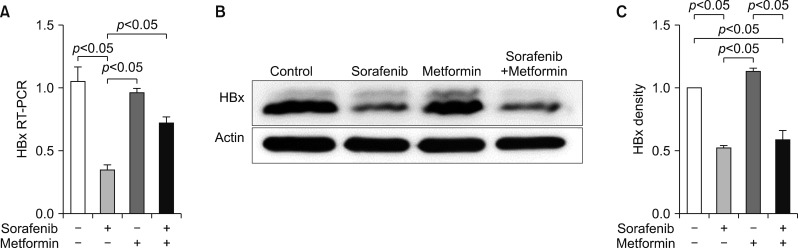
In contrast, RT-PCR revealed that metformin did not affect HBx expression; thus, no noticeable synergistic effect was present (Fig. 5).
Go to : 
Sorafenib is a multikinase inhibitor with therapeutic efficacy against HCC.16 Although sorafenib markedly prolongs the survival of patients with advanced HCC,456 its potential mechanisms of action that induce clinical benefits are yet to be properly elucidated. Metformin is a biguanide that inhibits cancer cell growth by inducing cell cycle arrest and enhancing apoptosis, and is also reportedly associated with a reduced risk of HCC.45678910
The mechanism of action of metformin is mainly associated with inhibition of the mTOR pathway, which plays an important role in the metabolism, growth, and proliferation of cancer cells.17 The inhibition of mTOR pathway is exerted by metformin via AMP-activated protein kinase (AMPK) activation, which is crucial for energy homeostasis.18 Metformin also inhibits the mTOR pathway in an AMPK-independent manner.14 AMPK-dependent and independent pathways are thought to be associated with the antitumor effects of metformin. Sorafenib can activate AMPK. A clinical study showed that patients with KRAS-mutant advanced non-small cell lung cancer receiving a combination of sorafenib and metformin had better outcomes than those receiving sorafenib alone. The study also showed that sorafenib and metformin act synergistically through inhibiting cellular proliferation in non-small cell lung cancer in vitro and in vivo and by phosphorylating the AMPK α activation site.19 Our in vitro study also demonstrated synergistic antitumor effects of both drugs. Thus, both sorafenib and metformin seem to activate the AMPK pathway, thus inducing synergistic antitumor effects.
In our in vitro study, we compared the potencies of the antitumor effects of metformin and sorafenib, in which the metformin-associated anti-tumor effect was variably comparable to that of sorafenib; however, sorafenib appeared to have a more powerful antitumor effect than metformin. The variability of response to the addition of metformin indicates that the combination of sorafenib and metformin treatment may lead to different therapeutic effects in a clinical setting.
In a clinical study with 93 patients treated with sorafenib, 31 has metformin administered concurrently due to diabetes mellitus. The concomitant use of sorafenib and metformin was associated with a median progression-free survival of 2.6 months, compared to 5.0 months for patients receiving sorafenib alone (p=0.029). The median overall survival of patients treated with the combination was 10.4 months, compared to 15.1 months for those who were not given metformin (p=0.014).20 These findings indicated increased tumor aggressiveness and resistance to sorafenib in metformin-treated patients, which is in direct contrast to the results of our in vitro study.
In conclusion, our in vitro study demonstrated the cytotoxic effects of metformin and synergistic antitumor effects with sorafenib. These results should be further verified in clinical studies with patients having advanced HCC.
Go to : 
ACKNOWLEDGEMENTS
Disclosure of source of funding: This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (Grant No 2015R1A2A2A04007141 to Shin Hwang and 2015K1A4A3046807 to Gi-Won Song).
Go to : 
References
1. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390. PMID: 18650514.

2. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim J, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009; 10:25–34. PMID: 19095497.

3. Davis E, Wiesner R, Valdecasas J, Kita Y, Rossi M, Schwartz M. Treatment of recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 2011; 17(Suppl 2):S162–S166. PMID: 21688382.

4. Ben Sahra I, Regazzetti C, Robert G, Laurent K, Le Marchand-Brustel Y, Auberger P, et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011; 71:4366–4372. PMID: 21540236.

5. Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007; 67:6745–6752. PMID: 17638885.

6. Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007; 67:10804–10812. PMID: 18006825.

7. Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006; 66:10269–10273. PMID: 17062558.

8. Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013; 62:606–615. PMID: 22773548.

9. Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010; 30:750–758. PMID: 20331505.

10. Lai SW, Chen PC, Liao KF, Muo CH, Lin CC, Sung FC. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol. 2012; 107:46–52. PMID: 22085817.

11. Kang WH, Tak E, Hwang S, Song GW, Jwa E, Lee YJ, et al. Metformin-associated chemopreventive effects on recurrence after hepatic resection of hepatocellular carcinoma: from in vitro to a clinical study. Anticancer Res. 2018; 38:2399–2407. PMID: 29599368.

12. Ling S, Song L, Fan N, Feng T, Liu L, Yang X, et al. Combination of metformin and sorafenib suppresses proliferation and induces autophagy of hepatocellular carcinoma via targeting the mTOR pathway. Int J Oncol. 2017; 50:297–309. PMID: 27959383.

13. Cheung PF, Yip CW, Ng LW, Lo KW, Chow C, Chan KF, et al. Comprehensive characterization of the patient-derived xenograft and the paralleled primary hepatocellular carcinoma cell line. Cancer Cell Int. 2016; 16:41. PMID: 27279800.

14. Kalender A, Selvaraj A, Kim SY, Gulati P, Brûlé S, Viollet B, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010; 11:390–401. PMID: 20444419.

15. Tak E, Hwang S, Lee HC, Ko GY, Ahn CS, Yoon YI, et al. Apoptosis of hepatitis b virus-expressing liver tumor cells induced by a high concentration of nucleos(t)ide analogue. Anticancer Res. 2016; 36:6059–6069. PMID: 27793933.

16. Ha TY, Hwang S, Moon KM, Won YJ, Song GW, Kim N, et al. Sorafenib inhibits migration and invasion of hepatocellular carcinoma cells through suppression of matrix metalloproteinase expression. Anticancer Res. 2015; 35:1967–1976. PMID: 25862849.
17. Chiang GG, Abraham RT. Targeting the mTOR signaling network in cancer. Trends Mol Med. 2007; 13:433–442. PMID: 17905659.

18. Gong L, Goswami S, Giacomini KM, Altman RB, Klein TE. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012; 22:820–827. PMID: 22722338.
19. Groenendijk FH, Mellema WW, van der Burg E, Schut E, Hauptmann M, Horlings HM, et al. Sorafenib synergizes with metformin in NSCLC through AMPK pathway activation. Int J Cancer. 2015; 136:1434–1444. PMID: 25080865.

20. Casadei Gardini A, Marisi G, Scarpi E, Scartozzi M, Faloppi L, Silvestris N, et al. Effects of metformin on clinical outcome in diabetic patients with advanced HCC receiving sorafenib. Expert Opin Pharmacother. 2015; 16:2719–2725. PMID: 26513009.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download