Dear Editor:
Sir, Basal cell carcinoma (BCC) is the most common malignant tumor of the skin. BCC is locally invasive and highly destructive, but rarely metastasizes
1. Identification of the pathogenic mechanisms underlying BCC could facilitate treatment and prevention of this cancer. It is generally accepted that BCC arises from keratinocyte stem cells
2, but its biological mechanisms, including those of its carcinogenesis, remain unknown. Leucine-rich repeat-containing G-protein-coupled receptor (LGR4) is expressed in proliferating tissues, including stem cells
3. LGR4 was reported to be expressed in the epidermis and hair follicles of human skin
4. Recently, it was reported that LGR4 promoted skin carcinogenesis by mediating the activation of MEK1/ERK1/2 and Wnt/
β-catenin pathways in mouse model
5. To our knowledge, no study has addressed LGR4 expression in BCC. In this study, we investigated the expression and localization of LGR4 in BCC via immunohistochemical analysis.
This study was approved by the ethics committee of The Catholic University of Korea (no. XC14SIMI0060K). All patients gave written informed consent. A total of 46 biopsy specimens that had been diagnosed as BCCs were collected from the three branch hospitals of The Catholic University of Korea. Paraffin sections of BCCs (total, n=47: including nodular [n=19], superficial [n=10], micronodular [n=4], metatypical [n=5], infiltrative [n=6], and nodular-infiltrative [n=3]) were analyzed for LGR4 expression.
The tissue sections were cut to 5 µm thickness. After deparaffinization and hydration, sections were subjected to the VECTASTAIN Elite ABC System and ImmPACT NovaRED (Vector Lab., Burlingame, CA, USA) according to the manufacturer's recommended procedure. Microscopic analysis was performed by two independent observers (ST Oh and JU Lee). The degree of expression was graded semi-quantitatively as follows: −, negative (0%); +, focal (1%~20%); ++, moderate (21%~50%); and +++, diffuse (>50%). LGR4 immunoreactivity was also assessed with respect to localization (membranous, cytoplasmic, or nuclear). The Mann-Whitney test was applied to compare LGR4 expression between histologically non-aggressive BCC and aggressive BCC. Additional Mann-Whitney tests with Bonferroni's correction were carried out for separate comparisons between non-aggressive BCC and the selected aggressive BCC subtype.
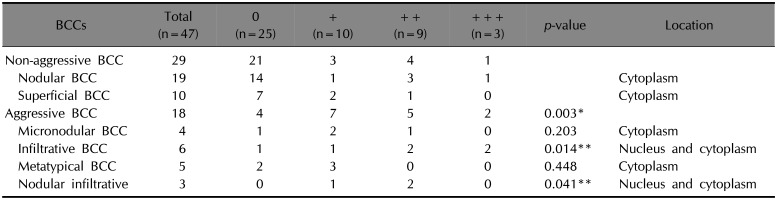
The expression of LGR4 in BCC is presented in
Table 1. Strong LGR4 immunoreactivity (++/+++) was detected in 14 of 46 cases (30%) of BCC. In histologically non-aggressive BCC (nodular BCC and superficial BCC), strong LGR4 immunoreactivity was detected in 5 of 29 cases (17%). In nodular (
Fig. 1A) and superficial BCC, LGR4 immunoreactivity was decreased compared to that in the overlying epidermis. In histologically aggressive BCC (micronodular BCC, infiltrative BCC, metatypical BCC, nodular-infiltrative BCC), strong LGR4 immunoreactivity was detected in 7 of 18 cases (39%). LGR4 expression was elevated in aggressive BCC as compared to that of non-aggressive BCC (
p=0.003). LGR4 expression in infiltrative BCC was elevated compared to that in non-aggressive BCC (
p<0.001) (
Fig. 1B). LGR4 expression in nodular-infiltrative BCC was elevated compared to that in non-aggressive BCC (
p=0.005) (
Fig. 1C, D).
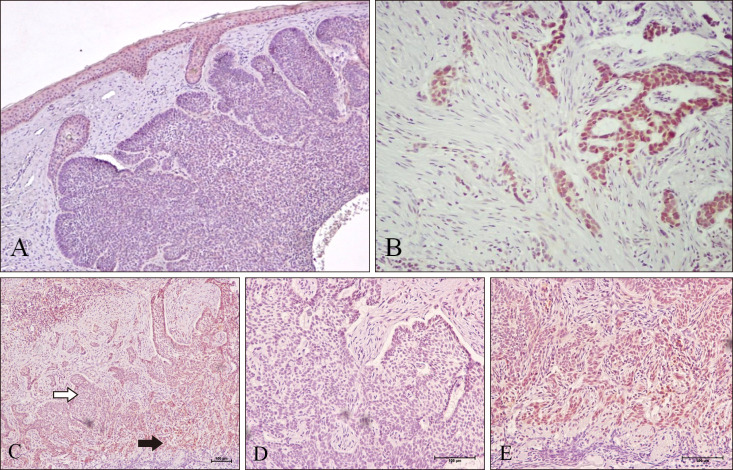 | Fig. 1(A) Notice decreased immunoreactivity in nodular BCC (×100). (B) Notice marked increased nuclear and cytoplasmic LGR4 expression in infiltrative BCC (×200) and (C) at the invasive front of nodular-infiltrative BCC (×100). The white arrow highlights the area magnified in (D) (×200). The black arrow highlights the area magnified in (E) (×200).
|
Table 1
LGR4 expression in BCCs
|
BCCs |
Total (n=47) |
0 (n=25) |
+ (n=10) |
++ (n=9) |
+++ (n=3) |
p-value |
Location |
|
Non-aggressive BCC |
29 |
21 |
3 |
4 |
1 |
|
|
|
Nodular BCC |
19 |
14 |
1 |
3 |
1 |
|
Cytoplasm |
|
Superficial BCC |
10 |
7 |
2 |
1 |
0 |
|
Cytoplasm |
|
Aggressive BCC |
18 |
4 |
7 |
5 |
2 |
0.003*
|
|
|
Micronodular BCC |
4 |
1 |
2 |
1 |
0 |
0.203 |
Cytoplasm |
|
Infiltrative BCC |
6 |
1 |
1 |
2 |
2 |
0.014**
|
Nucleus and cytoplasm |
|
Metatypical BCC |
5 |
2 |
3 |
0 |
0 |
0.448 |
Cytoplasm |
|
Nodular infiltrative |
3 |
0 |
1 |
2 |
0 |
0.041**
|
Nucleus and cytoplasm |

G protein-coupled receptors represent the largest group of cell surface receptors, and are currently used in medicine as therapeutic targets. They are involved in signal transduction systems and are particularly relevant in tumor cell biology. LGR4, also called G-protein-coupled receptor 48, is specifically expressed in stem cells of the epidermis and hair follicles
4. Overexpression of LGR4 has been reported in several types of cancer. It is well known that LGR4 activates Wnt/
β-catenin signaling to regulate cancer cell proliferation
6. LGR4 increased nuclear
β-catenin accumulation, and activated T-cell factor 4 transcription activity and expression of its target genes (including Cyclin D1, c-Myc, MMP-1 and MMP-7) that are important regulators of proliferation, cell cycle, migration, and invasion
7.
The histologic subtype of BCC influences the biologic behavior. BCCs with aggressive histologic patterns are commonly associated with recurrence after treatment. Based on this, BCC has been classified as histologically aggressive or non-aggressive, and nodular BCC is the main non-aggressive subtype. Traditionally, superficial BCC is regarded as non-aggressive due to its indolent behavior. Therefore, we suggest that superficial BCC should be included in the non-aggressive group. Sexton et al.
8 classified BCC as nodular, superficial, micronodular, infiltrative, morpheic pattern and a mixed pattern. Mixed BCC can be various, and so the nodular-infiltrative subtype in this study can be described as mixed BCC. Mixed BCC is now regarded as a histologically aggressive because of its aggressive growth pattern in a deeper potion of the tumor
9. Although, the significance of basosquamous carcinoma is still debatable, there is evidence that metatypical BCC (BCCs associated with moderate/severe squamous atypia) are associated with a higher incidence of recurrence and metastatic potential. Therefore, we suggest that aggressive BCC include infiltrative BCC, micronodular BCC, nodular-infiltrative BCC, and metatypical BCC.
LGR4 positively regulates Sonic Hedgehog (Shh) through unknown mechanisms in early prostate development
7. Shh signaling is a major signal transduction pathway in the pathogenesis of BCC. In addition, given that BCC arises from keratinocyte stem cells
1, and that LGR4 is a marker of stem cells in the epidermis and hair follicles
3, it is possible that LGR4 may play a role in the formation of BCC. BCC has been classified as aggressive and non-aggressive subtypes. In our study, we did not found little LGR4 expression in non-aggressive BCC. Therefore, it is conceivable that LGR4 may have little involvement in the development of non-aggressive BCC. Instead, we found that LGR4 expression was increased in aggressive BCC as compared to that of non-aggressive BCC. In addition, we detected strong LGR4 expression in the nucleus of tumor cells in infiltrative BCC and nodular-infiltrative BCC.
We previously reported that expression of beta-catenin was increased in aggressive BCC but not in non-aggressive BCC
10. It was postulated that LGR4 expression was positively associated with
β-catenin expression
7. Therefore, we speculate that increased expression of LGR4 in the aggressive BCC may be related with
β-catenin. Given that either
β-catenin or LGR4 contribute to promote migration and invasion of cancer cells
7, we suggest that LGR4 may play a role in cancer cell invasion of aggressive BCC.
In conclusion, we found LGR4 expression in aggressive BCC especially in infiltrative and nodular-infiltrative subtype, suggesting that LGR4 may play a role in the cancer cell invasion of BCC.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download