Abstract
Purpose
Primary care providers harbor misconceptions regarding penile prosthetic surgery, largely overestimating the rate of infection. Rates of infection following surgery for primary placement and revision are estimated as 1% to 3% and 10% to 18%, respectively. Our objective was to determine the contemporary incidence of infection following inflatable penile prostheses surgery at an academic training center where surgeons-in-training are routinely involved.
Materials and Methods
Review of a prospectively collected single-surgeon database was performed. All cases of inflatable penile prostheses placement from January 2011 through June 2017 were reviewed. Information regarding training level of assistant surgeon(s) was collected, and follow-up data was compiled regarding postoperative infections and need for revision surgery.
Results
Three hundred nine cases meeting inclusion criteria were identified. Mean patient age was 64.2 years, and mean follow-up was 28.7 months. Distribution involved 257 (83.2%) for primary placement, 45 (14.6%) for removal/replacement, and 7 (2.3%) in setting of prior device removal. Diabetes was noted in 31.1% of men. Surgeon-in-training involvement was noted in 100% of cases. Infection was confirmed in a patient who had skin breakdown over an area of corporal reconstruction with polytetrafluoroethylene. The overall postoperative infection rate was 0.3%.
Conclusions
In this series from an academic training center, infection following penile prosthetic surgery is low, similar to other centers of excellence, even with 100% involvement of surgeons-in-training. This data should be used to better inform primary care providers and members of the general public potentially interested in restoration of sexual function.
The prevalence of erectile dysfunction (ED) among men aged 40 to 70 exceeds 50% [1]. The inflatable penile prosthesis (IPP) is widely accepted as a safe and effective treatment for ED refectory to medication [2]. Device-mediated restoration is associated with patient satisfaction in excess of 90% [3]. While infection remains a feared complication, as it often demands reoperation and can increase cost of care as much as six-fold [4], the incidence appears highly overestimated among various members of the medical community. The infection rate following primary device insertion is only 1% to 3%, while some single-surgeon studies have reported rates as low as 0.46% when using coated implants and the ‘no touch’ technique [5]. Rates following revision surgery are reportedly higher at 7% to 18% [6]. However, a recent survey of primary care physicians (PCPs) highlighted deficient awareness relative to this data. The 77% believed the patient satisfaction rate was around 30%, and 62% said they would not refer patients for IPP due to a perceived infection rate of >25% [7]. Additionally, misguided surgeon-authored texts sprinkled with poorly-informed editorial commentary demonizing IPPs as inappropriate therapy with excessive complications still make their way into the hands of medical students and residents [8].
Men who would stand to gain from erectile restoration may fear surgery at academic centers due to a perceived operative risk from involvement of residents and/or fellows. It has been reported that patients undergoing operations on reproductive or sexual organs were more apprehensive of resident involvement [9]. Within the urologic literature, the data is mixed as to whether resident involvement increases complications [1011]. However, this topic has not been well studied in device-mediated erectile restoration. Given the perceptions among the medical and general community regarding involvement of surgeons-in-training and rates of infection following IPP surgery, we chose to evaluate the contemporary incidence of postoperative infection at an academic training center.
We retrospectively reviewed a prospectively collected, Institutional Review Board-approved database of urologic prosthetic surgery performed by a single reconstructive urologist at Wake Forest University Baptist Medical Center (Winston-Salem, NC, USA) (approval number: 00042919). We identified patients who had undergone an IPP-related surgery (i.e., primary placement, removal and replacement, or delayed replacement) from January 2011 through June 2017. All patients were included in the study.
Pre-, intra-, and postoperative protocols were consistent throughout the study period. All patients were required to hold blood-thinning and antiplatelet agents for ten days preoperatively and hemoglobin A1c (HbA1c) levels were required to be below 9% prior to surgery. Patients were instructed to wash their genitals with chlorhexidine gluconate soap the night before and the morning of surgery. A 10 minute chlorhexidine scrub was routinely utilized, and an iodine impregnated drape was used to cover most of the scrotal skin. Patients were given vancomycin and gentamicin prior to incision and these medications were continued for 24 hours postoperatively. Patients were discharged home with two weeks of antibiotics (typically trimethoprim/ sulfamethoxazole unless contraindicated).
Surgical technique was consistent across the studied interval. The attending surgeon performed all key and critical portions of the operation with the surgeons-intraining assisting in all cases. All reservoirs were placed in the retropubic space, even in those with prior robotic prostatectomy. No patient received a drain or compressive dressing.
Data related to patient demographics were collected and analyzed. Inpatient and outpatient notes were reviewed for peri- and postoperative follow-up information, with special attention to patients presenting with symptoms concerning for prosthetic infection. Operative notes were reviewed for surgical technique, postgraduate year (PGY) of assistant surgeon(s), and intraoperative observations and diagnoses. The reconstructive urology fellow was considered a PGY-6.
Three hundred nine IPP cases were identified. Patient demographics are summarized in Table 1. The average patient was a senior citizen, nearly a third had diabetes, and obesity was common. The majority were either former or current tobacco users. Nearly all devices were threepiece inflatable models (of both major manufacturers in the United States), except for one two-piece device and one malleable. All inflatable devices were placed via a penoscrotal approach, except for one infrapubic case in a patient with a history of recurrent scrotal hidradenitis. Procedure type is summarized in Table 2, with most surgeries performed (83.2%) being a primary placement of an IPP.
Mean follow-up was 28.7 months. Surgeons-in-training were involved in 100% of cases (Table 3). Involvement distribution was as follows: 91 cases (29.4%) were assisted by a PGY 1 or 2, 26 (8.4%) by a PGY 3, 13 (4.2%) by a PGY 4, 150 (48.5%) by a PGY 5, and 170 (55.0%) by a PGY 6. Some cases involved multiple trainees, accounting for the total involvement distribution exceeding 100%. The rate of clinical infection in this series was 0.3% (1/309 patients). The patient requiring removal for clinical infection had a complicated history. He was referred after undergoing initial surgery at an outside center, at which time a semirigid device had been placed for ED and Peyronie's disease through a circumcising incision. He reportedly had a thermal injury and later presented with penile gangrene of the left side of the penis resulting in device removal and suggestion of urethral fistula. Nearly a year afterward, he underwent IPP placement at Wake Forest Baptist Medical Center with concomitant urethroplasty and corporoplasty using allograft. He was managed with a single cylinder as the left corpora was obliterated distally, and later received a glanuloplasty for what was felt to be impending extrusion. Over a year later, he requested a contralateral cylinder, and a new IPP was placed with polytetrafluoroethylene-based reconstruction of the left side. Subsequent skin breakdown resulted in obvious clinical infection, and the device was removed nearly 6 weeks later. He has required two tissue rearrangements by plastic surgery and is still without a penile implant.
Of note, one patient in this series had pain at his pump site 5.5 months after device placement. Clinical examination was normal and ultrasound was within normal limits. He underwent removal with replacement with a downsized device, and pain subsequently resolved. No purulence was noted at time of surgery and intraoperative cultures were negative. He has been without pain for over 18 months of subsequent follow-up. A third patient with a history of diabetes underwent device removal for fever of unknown origin three weeks following IPP placement. Clinical examination was normal and cross sectional imaging was negative for obvious pathology. Intraoperative culture at time of device removal was negative. Fever persisted and was deemed secondary to pneumonia less than a week later, and resolved following appropriate treatment.
Given an increased focus on quality metrics for hospital and physician reimbursement, there is increased pressure on surgical attendings charged with training residents and fellows to provide an adequate operative experience while maintaining the highest quality, cost-conscious care. Particularly specialized fields with high operative demands, such as reconstructive urology have the potential to witness decreased operative involvement for residents. Relevant factors may include the requirement of direct supervision, the reported expense of teaching in the operating room, potential prolongation of operative time secondary to resident involvement, in addition to the perception that surgeon-in-training involvement yields higher complication rates [11].
To our knowledge, this study is the first to quantify and demonstrate unanimous involvement of surgeons-in-training while evaluating the associated infection rates following IPP surgery at a high-volume academic center. Despite 100% involvement of trainees in IPP surgery, the rate of infection at this institution was comparable to other published series [5]. Importantly, the attending surgeon performed all key and critical portions of the operation, so this must be considered when comparing the results from our institution with those at institutions where the trainees perform critical portions. Nonetheless, our results suggest that fears related to a potentially exaggerated infection risk in the setting of a training environment are likely unwarranted. Similarly, exaggerated estimates of risk by PCPs and/or surgeons who do not provide these operative services, regardless if such misinformation is being delivered to patients or medical professionals, appears to be inappropriate and irresponsible [8].
The general public has strong concerns about resident performance, and is increasingly aware of issues related to work hours and risk of errors, as well as the controversy regarding overlapping cases [12]. In one study, 70.3% of patients presenting to a teaching hospital for surgery were found to have significant preoperative anxiety, and after a fear of postponement, the number one fear was medical mistakes resulting in harm [13]. General apprehension may be fed, in part, by surgeons in the private sector promoting the concept that patients should avoid elective surgery at teaching hospitals. Surgeons who support resident involvement have documented objections from patients who, at times, have even stressed the quality of their insurance as a reason why they should not have a trainee involved in their operation [14]. Additionally, some surgeons have asserted that influx of new residents at the beginning of the academic year (also known as the ‘July phenomenon’) is associated with poorer outcomes at teaching hospitals [15]. Major periodicals have advised members of the public considering surgery at a teaching hospital to ask who specifically performs the operation, and which parts may involve residents since ‘inexperienced surgeons… are learning how to perform surgery—that's how the system works’ [16].
The urologic literature contains mixed reports as to the influence of resident involvement upon rates of operative complications. A large study utilizing the National Surgical Quality Improvement Program (NSQIP) Database found that resident and fellow involvement in renal surgery was associated with higher superficial and overall surgical site infection (SSI) (p<0.05). In addition, PGY 6 resident and fellow involvement was associated with higher organ space infections and overall SSI (p<0.05) [17]. Several other studies in our field, however, have failed to demonstrate an increased risk of complications relative to learner participation [1118]. In our series, albeit with a different operation, an infection rate much lower than the average cited in the literature (0.3%) was noted despite 100% involvement of surgeons-in-training. It is possible that the success noted in our experience was, in part, due to involving these trainees, rather than despite their presence. In fact, another study of urologic surgery using NSQIP data noted a protective effect of resident involvement [10].
The patients comprising this study population were not without risk factors for infection. Previously identified conditions that may elevate the chance for postoperative infection include diabetes, immunosuppression, urinary tract infection (UTI), and spinal cord injuries [1920]. Although the data is mixed as to whether HbA1c and fasting glucose levels correlate with an increased risk of IPP infection [21], the increased risk among diabetics overall is well documented [1922]. Diabetics comprised nearly one-third of our patient population, yet the outcomes were excellent. To better understand the relationship between preoperative A1c and clinical outcomes after major non-cardiac surgery, Underwood et al. [23] reviewed data from NSQIP and the Research Patient Data Registry of the Brigham and Women's Hospital. The study's primary outcome was length of stay (LOS) as infection outcomes were too small for comprehensive analyses. A1c >8% was associated with increased LOS and may be a surrogate for poorer surgical outcomes. British guidelines have previously recommended an arbitrary cutoff of 8.5% [24]. However, some have suggested that thresholds of <8% may not be ideal for many people, particularly the elderly, based on a risk of developing hypoglycemia [25]. As such, we maintain a requirement that A1c values are <9% prior to elective surgery.
Although there is a paucity of data unique to urologic surgery, smoking and obesity are both recognized surgical risk factors. A large meta-analysis determined the risk for surgical infection among smokers as 1.79 times that of non-smokers. Additionally, former smokers also carry an increased risk versus individuals who have never smoked [26]. Most of our patients were current or former smokers. Obesity has also been linked to increased risk of SSI and UTI [2728]. Obese or morbidly obese patients accounted for 45% of our patient population. Thus, even in the setting of multiple risk factors, infection following IPP surgery remains reasonably low.
Some providers may be reluctant to advocate erectile restoration for elderly patients based on presumption of age-associated risk. 10% of the men in this series were age 75 or greater at the time of surgery. With aging, there is a recognized functional decline in both innate and adaptive immune response [29], predisposing elderly patients to infection. The one prior study on post-IPP infections among the elderly found no infections among the 30 patients aged ≥75 [30]. Our study reinforces this data, showing no infections in our 31 patients aged ≥75. As such, we do not employ any age-specific cutoffs for consideration of erectile restoration, instead choosing to focus on the overall health of the patient.
This study is not without limitations. There was no control arm (IPP surgery performed without a surgeon-intraining) with which to compare infection rates. There was also no gauge to quantify extent of maneuvers performed by residents based on review of operative notes. Surgeonin- training participation is likely influenced by multiple factors including the trainee's interest and initiative. Furthermore, while a mean in-clinic follow-up of 28.7 months may capture many potential issues, we recognize that infections can present at a much later time, such that longer follow-up is warranted. Additionally, some patients may present to outside centers for infectious complications, which would limit data capture. Future efforts could involve periodic phone calls to patients released from practice after years of uncomplicated follow-up, or possibly partnering with industry to query patients through their database. Also, negative cultures at revision surgery for clinically uninfected cases may not be enough to fully rule out presence of infection. Admittedly, the low infection rate in this series may not be reflective of all centers. The surgeon in this series is fellowship-trained and maintains a high-volume practice, which may translate to better outcomes. Additionally, 49% of the cases in this dataset were assisted by a PGY 5 and 55% by a PGY 6, and the attending surgeon was present and performed for all key and critical portions of the operation. Another limitation involves the absence of data in this series relative to operative time. It is logical to assume that trainee involvement may extend operative time, which certainly has implications for cost of care. Furthermore, patient selection is relevant to outcomes. Even though diabetics comprised a significant percentage of the study population, we maintain a preoperative A1c requirement (<9%). The need for clearance to hold bloodthinning medications may also bias our series towards men with better cardiovascular health than those managed by surgeons willing to perform surgery while patients are actively using medications, such as aspirin.
As ED can have a significant negative impact upon quality of life, it is important for PCPs and the general public to be well informed on the available therapeutic options and the associated risks and benefits. Data demonstrate that a substantial number of these individuals harbor poorly-informed perspectives on procedures involving surgeons-in-training and the true risk of infection following penile prosthesis placement. Our data demonstrate that these operations can be performed in an academic center with excellent outcomes. Future work to better educate referring providers and men/couples at risk for sexual health concerns seems warranted.
Notes
CONFLICTS OF INTEREST: Dr. Amy M. Pearlman is a consultant for American Medical System/Boston Scientific. Dr. Ryan P. Terlecki is a consultant for and receives grant funding from American Medical Systems/Boston Scientific. These conflicts of interest have, in no way, influenced the results of our work. Dr. Kara E. McAbee has no conflicts of interest to disclose.
References
1. Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994; 151:54–61. PMID: 8254833.

2. Levine LA, Becher E, Bella A, Brant W, Kohler T, Martinez-Salamanca JI, et al. Penile prosthesis surgery: current recommendations from the international consultation on sexual medicine. J Sex Med. 2016; 13:489–518. PMID: 27045255.

3. Trost LW, McCaslin R, Linder B, Hellstrom WJ. Long-term outcomes of penile prostheses for the treatment of erectile dysfunction. Expert Rev Med Devices. 2013; 10:353–366. PMID: 23668707.

4. Holland B, Kohler T. Minimizing penile implant infection: a literature review of patient and surgical factors. Curr Urol Rep. 2015; 16:81. PMID: 26480830.

5. Eid JF, Wilson SK, Cleves M, Salem EA. Coated implants and “no touch” surgical technique decreases risk of infection in inflatable penile prosthesis implantation to 0.46%. Urology. 2012; 79:1310–1315. PMID: 22521187.

6. Henry GD, Wilson SK. Updates in inflatable penile prostheses. Urol Clin North Am. 2007; 34:535–547. vi. PMID: 17983893.

7. Mishail A, Maggio-Ferguson A, Lee W, D'Amato A. Perception vs reality: how primary care physicians understand prosthetic urology. J Urol. 2016; 195:e630.

8. Pestana C. Dr. Pestana's surgery notes. 2nd ed. New York: Kaplan Publishing;2015.
9. Carruthers KH, McMahan JD, Taylor A, Pearson G, Tiwari P, Kocak E. Patient attitudes toward resident participation in cosmetic vs reconstructive outpatient consultations. J Surg Educ. 2015; 72:477–482. PMID: 25572941.

10. Matulewicz RS, Pilecki M, Rambachan A, Kim JY, Kundu SD. Impact of resident involvement on urological surgery outcomes: an analysis of 40,000 patients from the ACS NSQIP database. J Urol. 2014; 192:885–890. PMID: 24704012.

11. Schommer E, Tonkovich K, Li Z, Thiel DD. Impact of resident involvement on robot-assisted radical prostatectomy outcomes. J Endourol. 2016; 30:1126–1131. PMID: 27445128.

12. Kent M, Whyte R, Fleishman A, Tomich D, Forrow L, Rodrigue J. Public perceptions of overlapping surgery. J Am Coll Surg. 2017; 224:771–778.e4. PMID: 28196693.

13. Nigussie S, Belachew T, Wolancho W. Predictors of preoperative anxiety among surgical patients in Jimma University Specialized Teaching Hospital, South Western Ethiopia. BMC Surg. 2014; 14:67. PMID: 25189274.

14. Wu J. You should want surgery residents for your operation. Here's why [Internet]. Kevin MD;2016. cited 2016 Apr 19. Available from: https://www.kevinmd.com/blog/2016/04/you-should-want-surgery-residents-for-your-operation-heres-why.html.
15. Jena AB, Sun EC, Romley JA. Mortality among high-risk patients with acute myocardial infarction admitted to U.S. teaching-intensive hospitals in July: a retrospective observational study. Circulation. 2013; 128:2754–2763. PMID: 24152859.
16. Webster H. Is surgery safer at a teaching hospital? [Internet]. US News;2014. cited 2014 Oct 27. Available from: https://health.usnews.com/health-news/patient-advice/articles/2014/10/27/is-surgery-safer-at-a-teaching-hospital.
17. Kern SQ, Lustik MB, McMann LP, Thibault GP, Sterbis JR. Comparison of outcomes after minimally invasive versus open partial nephrectomy with respect to trainee involvement utilizing the American College of Surgeons National Surgical Quality Improvement Program. J Endourol. 2014; 28:40–47. PMID: 24007345.

18. Ruhotina N, Dagenais J, Gandaglia G, Sood A, Abdollah F, Chang SL, et al. The impact of resident involvement in minimally-invasive urologic oncology procedures. Can Urol Assoc J. 2014; 8:334–340. PMID: 25408800.

19. Jarow JP. Risk factors for penile prosthetic infection. J Urol. 1996; 156:402–404. PMID: 8683689.

20. Selph JP, Carson CC 3rd. Penile prosthesis infection: approaches to prevention and treatment. Urol Clin North Am. 2011; 38:227–235. PMID: 21621089.

21. Cakan M, Demirel F, Karabacak O, Yalçinkaya F, Altuğ U. Risk factors for penile prosthetic infection. Int Urol Nephrol. 2003; 35:209–213. PMID: 15072498.

22. Mulcahy JJ, Carson CC 3rd. Long-term infection rates in diabetic patients implanted with antibiotic-impregnated versus nonimpregnated inflatable penile prostheses: 7-year outcomes. Eur Urol. 2011; 60:167–172. PMID: 21316145.

23. Underwood P, Askari R, Hurwitz S, Chamarthi B, Garg R. Preoperative A1C and clinical outcomes in patients with diabetes undergoing major noncardiac surgical procedures. Diabetes Care. 2014; 37:611–616. PMID: 24170760.

24. Dhatariya K, Levy N, Kilvert A, Watson B, Cousins D, Flanagan D, et al. NHS Diabetes guideline for the perioperative management of the adult patient with diabetes. Diabet Med. 2012; 29:420–433. PMID: 22288687.

25. Dhatariya K. Comment on Underwood. . Comment on Underwood et al. Preoperative A1C and clinical outcomes in patients with diabetes undergoing major noncardiac surgical procedures. Diabetes Care. 2014; 37:611–616. e190. PMID: 24170760.
26. Sørensen LT. Wound healing and infection in surgery. The clinical impact of smoking and smoking cessation: a systematic review and meta-analysis. Arch Surg. 2012; 147:373–383. PMID: 22508785.
27. Choban PS, Heckler R, Burge JC, Flancbaum L. Increased incidence of nosocomial infections in obese surgical patients. Am Surg. 1995; 61:1001–1005. PMID: 7486411.
28. Huttunen R, Karppelin M, Syrjänen J. Obesity and nosocomial infections. J Hosp Infect. 2013; 85:8–16. PMID: 23920442.

29. Wong C, Goldstein DR. Impact of aging on antigen presentation cell function of dendritic cells. Curr Opin Immunol. 2013; 25:535–541. PMID: 23806201.

30. Chung E, Solomon M, DeYoung L, Brock GB. Clinical outcomes and patient satisfaction rates among elderly male aged ≥75 years with inflatable penile prosthesis implant for medically refractory erectile dysfunction. World J Urol. 2014; 32:173–177. PMID: 23708698.
Table 1
Patient demographics (n=309)
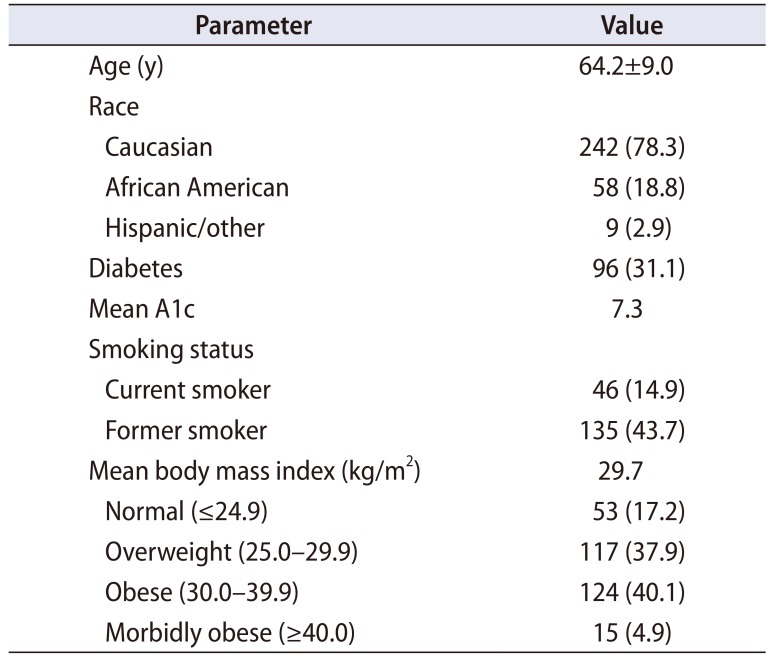
Table 2
Nature of surgical intervention (n=309)
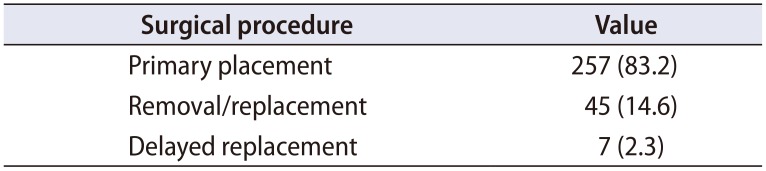
| Surgical procedure | Value |
|---|---|
| Primary placement | 257 (83.2) |
| Removal/replacement | 45 (14.6) |
| Delayed replacement | 7 (2.3) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download