Abstract
Purpose
Ureteroscopic lithotripsy (URS) is gaining popularity for the management of ureteral stones and even renal stones, with high efficacy and minimal invasiveness. Although this procedure is known to be safe and to have a low complication rate, febrile urinary tract infection (UTI) after URS is not rare. Therefore, we aimed to analyze the risk factors and causative pathogens of febrile UTI after URS.
Materials and Methods
Between January 2013 and June 2015, 304 patients underwent URS for ureteral or renal stones. The rate of postoperative febrile UTI and the causative pathogens were verified, and the risk factors for postoperative febrile UTI were analyzed using logistic regression analysis.
Results
Of 304 patients, postoperative febrile UTI occurred in 43 patients (14.1%). Of them, pathogens were cultured in blood or urine in 19 patients (44.2%), and definite pathogens were not identified in 24 patients (55.8%). In patients with an identified pathogen, Pseudomonas aeruginosa had the highest incidence. Multivariate analysis showed that the operation time (p<0.001) was an independent risk factor for febrile UTI after URS. The cut-off value of operation time for increased risk of febrile UTI was 70 minutes.
Conclusions
Overall, febrile UTI after URS occurred in 14.1% of patients, and the operation time was an independent predictive factor for this complication. Considering that more than 83.7% of febrile UTIs after URS were not controlled with fluoroquinolones, it may be more appropriate to use higher-level antibiotics for treatment, even in cases with unidentified pathogens.
Technology has brought many advantages to ureteroscopy for the treatment of urinary stones, including decreased procedural invasiveness and a high success rate. In recent times, ureteroscopic lithotripsy (URS) is widely used, not only for ureteral stones, but also for renal stones. The first flexible ureteroscopic procedures were introduced in the 1960s [123]. With the development of a smaller diameter, increased flexibility, and digital ureteroscopes, flexible URS has become one of the standard procedures for the management of small or mid-sized renal stones. It has shown high stone-free success levels and acceptable complication rates [4].
Recent reports indicate that the overall complication rate of ureteroscopic procedures ranges from 9% to 25% [56789]. Although urinary tract infections (UTIs), ureteral injuries, hematuria, and postoperative renal colic have been reported as common complications of ureteroscopic procedures, UTI is one of the most frequent complications [5671011]. To prevent procedure-related infection, prophylactic antibiotics are routinely administered, even in endoscopic surgery. Traditionally, fluoroquinolones have been used as prophylactic antibiotics for URS; however, febrile UTI following the procedure is not rare even with prophylactic antibiotics. However, only a few clinical studies researching the pathogens of febrile UTI af ter URS have been conducted, and the overall understanding of postoperative febrile UTI and appropriate antibiotic therapy is limited. Therefore, this study aimed to analyze the characteristics and risk factors of febrile UTI after URS and endeavored to seek the most effective prevention and management methods.
The Institutional Review Board of the Kyungpook National University Hospital approved the study protocol based on the Declaration of Helsinki (approval number: KNUH 2018-03-014). Between January 2013 and June 2015, 304 patients who underwent URS for the management of ureteral and/or renal stones were included in this retrospective study. Preoperative urine cultures were performed, and patients with preoperative UTI (pyuria or bacteriuria) were excluded from this study. Perioperative clinical information included: age, gender, body mass index, underlying diseases (hypertension, diabetes mellitus, chronic renal failure), previous stone history (primary or recurrent), stone characteristics (number of stones, stone size, multiplicity, laterality, location), methods of URS (semirigid/flexible/combined), presence of hydronephrosis on preoperative image, preoperative ureteral stent, preoperative percutaneous nephrostomy, and operation time. All patients underwent a non-contrast computed tomography scan to evaluate the stone characteristics. In case of multiple stones, the stone size was calculated as the sum of the diameters of each stone.
A fluoroquinolone was prophylactically administered 1 hour before surgery, and oral antibiotics were administered for 5 days postoperatively. URS was performed with a semirigid ureteroscope (8.5-Fr; Karl Storz, Tuttlingen, Germany) and/or a flexible ureteroscope (8.2-Fr, URF-P5; Olympus, Tokyo, Japan). After cystoscopy, a hydrophilic guidewire was advanced vertically inside the ureter under fluoroscopy guidance. In cases of flexible URS, a ureteral access sheath (12/14-Fr; Cook Urological Inc., Bloomington, IN, USA) was inserted along the guidewire up to the proximal ureter. Stones were fragmented using a 200-µm holmium laser lithotripter (Lumenis Inc., Tel Aviv, Israel). After the procedure, large fragments of calculi were removed with a 1.9-Fr nitinol stone basket (Zero-tip; Boston Scientific, Marlborough, MA, USA), whereas small fragments (less than 2 mm in diameter) were left for natural drainage. At the end of the procedure, a 6-Fr double-J ureteral stent was inserted in all patients to reduce postoperative flank pain. Procedural success was defined as no obvious stones or stones less than 2 mm according to simple X-ray of the kidney, ureter, and bladder or ultrasound examination at 2 weeks after the URS. A postoperative febrile UTI was defined as the occurrence of a fever higher than 38℃ combined with pyuria within 1 week of surgery and without any infectious signs in other organs. The postoperative febrile UTI incidence and the causative pathogens were verified, and risk factors were analyzed with logistic regression analysis. Multivariate analysis was used to identify the risk factors of postoperative febrile UTI. Statistical analyses were performed using PASW Statistics ver. 18.0 software (IBM Co., Armonk, NY, USA), and p-value <0.05 was considered statistically significant.
The overall incidence of febrile UTI was 14.1% (43/304 patients). Table 1 shows the patients' demographics and stone characteristics. The mean patient age was 56.9±13.1 years, and the mean stone size was 10.0±4.1 mm. Of the 304 patients, 207 patients (68.1%) were male and 97 patients (31.9%) were female. The underlying diseases included hypertension (42.8%), diabetes mellitus (23.7%), and chronic kidney disease (5.3%). The mean body mass index was 25.1±3.8 kg/m2. A total of 48 patients (15.8%) had a history of stone surgery, and 148 patients (48.7%) had preoperative hydronephrosis. Three patients (1.0%) had preoperative ureteral stents, and 55 patients (18.1%) had percutaneous nephrostomies. The mean operation time was 67.1±27.9 minutes.
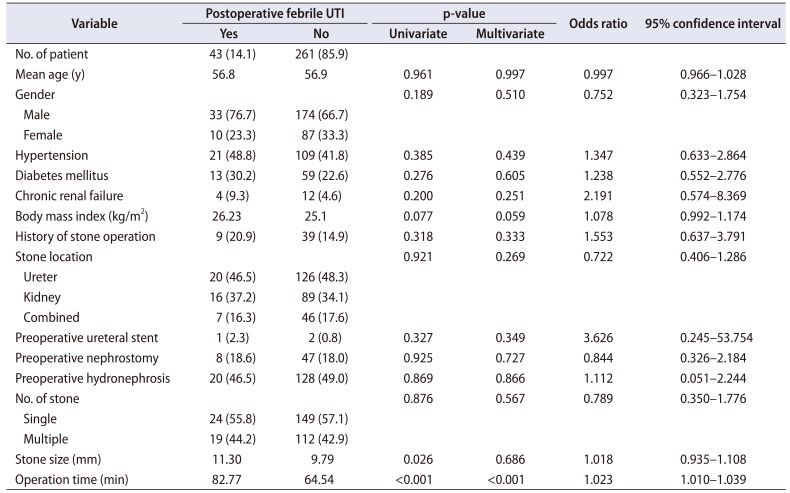
Table 2 shows the comparison of clinical information between the febrile UTI group and the non-febrile UTI group. Univariate analysis showed that the stone size (p=0.026) and the operation time (p<0.001) were significantly different between the febrile UTI and non-febrile UTI groups, whereas patients' age, gender, body mass index, underlying diseases, presence of hydronephrosis, multiple stones, previous history of stone surgery, presence of preoperative ureteral stent or percutaneous nephrostomy, and location of stones were not significantly different between the two groups. In this study, 146 patients underwent URS, 105 patients underwent retrograde intrarenal surgery (RIRS), and 53 patients underwent URS with RIRS. No difference in the incidence of postoperative UTI was detected according to surgical modality (p=0.921). In the multivariate logistic model, only the operation time was independently correlated with postoperative febrile UTI (odds ratio, 1.023; 95% confidence interval, 1.010–1.039; p<0.001). Receiver operating curve analysis showed that the cut-off value of the operation time that could predict a high risk of febrile UTI after URS was 70 minutes (sensitivity: 58.1%, specificity: 61.7%).
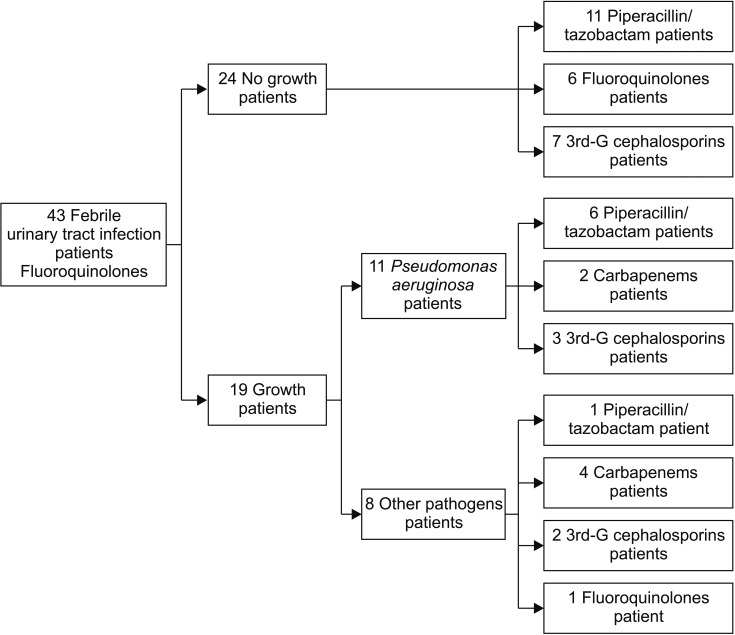
Of 43 patients with a febrile UTI, true pathogens were cultured in blood or urine in 19 patients (44.2%), and definite pathogens were not cultured in 24 patients (55.8%). Pseudomonas aeruginosa was the most commonly cultured in 11 patients (25.6%), and Escherichia coli, Staphylococcus capitis, S. epidermidis, Proteus mirabilis, Enterococcus faecalis, Enterobacter cloacae, Citrobacter amalonaticus, and Achromobacter xylosoxidans were cultured in 1 patient (2.3%) each.
Fig. 1 shows the algorithm of administered antibiotics. In all 43 patients with a febrile UTI, fluoroquinolones were initially administered until pathogens were cultured. Of 19 patients with a trigger pathogen identified in their blood and/or urine culture, antibiotics were changed to piperacillin/tazobactam in 7 patients, a 3rd generation cephalosporin in 5 patients, and a carbapenem in 6 patients, while 1 patient remained on fluoroquinolones. Of the 24 patients without an identified causative pathogen in their blood and/or urine culture, 11 were managed with piperacillin/tazobactam, 7 patients received a 3rd generation cephalosporin, and 6 patients were maintained on fluoroquinolones. After appropriate antibiotics administration, the condition of all the patients improved.
Prophylactic antibiotics are commonly used to prevent infection in urological procedures performed for conventional diagnostic and therapeutic purposes. Many studies have been conducted to investigate the use of prophylactic antibiotics for URS, and their use has been proven to reduce the occurrence of postoperative fever and UTI. According to the European Association of Urology Guidelines, f luoroquinolones are recommended as prophylactic antibiotics for diagnostic ureteroscopy and URS. The American Urological Association Best Practice Guidelines [12] also suggest that all patients undergoing URS should receive a prophylactic fluoroquinolone drug or trimethoprim-sulfamethoxazole. Knopf et al. [13] conducted a randomized study to compare single dose oral levofloxacin (250 mg) to no prophylactic antibiotics in URS. Their primary measurement was bacteriuria, which resulted in an incidence of 1.8% vs. 12.5%, respectively, a significantly lower incidence in the prophylactic antibiotics group.
In our study, the prophylactic antibiotics administered were fluoroquinolones. However, despite this, postoperative febrile UTI after URS was not rare, occurring in 14.1% of the patients at Kyungpook National University Hospital. Mitsuzuka et al. [14] analyzed 153 patients who underwent URS for renal and/or ureteral stones between 2011 and 2013, and reported that febrile UTI occurred in 18.3% of the patients. The major difference between our study and that of Mitsuzuka et al. [14] is the inclusion criteria. They included patients with preoperative pyuria, and this may be a reason for the higher incidence of febrile UTI. The study suggested that preoperative pyuria is a risk factor for febrile UTI. Sohn et al. [15] analyzed 531 patients who underwent diagnostic ureteroscopy or URS and reported that 3.8% of patients had infectious complications. This study showed a lower incidence of febrile UTI when compared to our study, which may be related to the inclusion of diagnostic ureteroscopy, which carries lower risk of infectious complications than the lithotripsy does. Our study showed that trigger pathogens were identified in only 19 of 43 patients with postoperative febrile UTI, which means that many patients did not have bacteremia or bacteriuria. Since most causative pathogens of nosocomial UTI are E. coli, Enterobacter spp., and P. aeruginosa [16], fluoroquinolones were empirically administered as the initial antibiotics until the pathogens were identified. If the pathogens were identified and the results of antimicrobial susceptibility were revealed through blood or urine culture, it was not difficult to determine the next therapeutic regimen.
According to clinical practice guidelines for the antibiotic treatment of community-acquired urinary tract infections [17], E. coli is the most common causative bacterium in UTI in Korea. In Korea, fluoroquinolones are mainly recommended as the primary antibiotic for UTIs. However, since the resistance to fluoroquinolones of causative bacteria of UTIs in Korea is higher than that reported in the United States and Europe, the failure rate of fluoroquinolone treatments is high. In 2006, the antibiotic resistance of E. coli isolated from cystitis to ciprofloxacin was 24.6% in Seoul, 40.0% in Gyeongsang Province, 14.7% in Gyeonggi Province, and 32.1% in Chungcheong Jeolla Province [18]. Therefore, the selection of empirical antibiotics should be based on data from different time periods and regions. However, E. coli, which induces UTIs in Korea, is highly resistant to ciprofloxacin, ampicillin/sulbactam, and gentamicin. Thus, for UTIs accompanied by sepsis or recurrent UTIs, piperacillin/ tazobactam, third- or fourth-generation cephalosporin, amikacin, and carbapenem are recommended as a priority. Some experts recommend antipseudomonal antibiotics in the early stage of severe infections suspected as sepsis or healthcare-associated infections. After beginning early empirical treatment using broad-spectrum antibiotics, the treatment should be readjusted according to obtained culture results [17]. In these patients, we gradually stepped up the antibiotic regimen. If the infection was well-controlled using fluoroquinolones and no definite pathogens were identified, patients received intravenous fluoroquinolone drugs until pyuria was rarely seen and inflammatory serum markers, such as leukocytosis and elevated C-reactive protein level, were normalized. However, if these patients showed persistent fever and uncontrolled UTI symptoms, we changed the antibiotics to a 3rd generation cephalosporin. Then, if the UTI was under control, the patients were maintained on these antibiotics; however, if the symptoms of UTI did not improve over 2 or 3 days, the antibiotics were changed to piperacillin/tazobactam.
In this study, the most frequent causative pathogen of febrile UTI was P. aeruginosa, which was identified in 11 patients (25.6%). This suggests that in the event of a postoperative febrile UTI, the possibility of infection by Pseudomonas should be considered, despite the use of prophylactic antibiotics, such as fluoroquinolones. Currently, the rate of fluoroquinolone-resistant P. aeruginosa in hospitals is increasing [19]. Therefore, if a postoperative febrile UTI is not well-controlled under the regimen of prophylactic antibiotics, an early step-up of antibiotics should be considered even before the results of culture and antimicrobial susceptibility are reported.
Several studies assessed the risk factors of febrile UTI after URS. Mitsuzuka et al. [14] demonstrated that preoperative pyuria and pyelonephritis were significant risk factors for postoperative febrile UTI. Sohn et al. [15] reported that bacteriuria and previous catheterization were the strongest risk factors of febrile complications. Grabe et al. [20] suggested that bacteriuria, preoperative ureteral stents, and percutaneous nephrostomy were the risk factors of febrile complications. Previous pyuria was excluded, and preoperative ureteral stenting was not common in our study. Although cases of percutaneous nephrostomy were included in our study due to previous pyelonephritis, all cases were managed for UTI before surgery.
Thirty-three of 207 male patients in this study had febrile UTI. Preoperative digital rectal examination was performed in all male patients and acute prostatitis was not suspected in any patient. In addition, all 33 patients who had febrile UTI did not have prostatitis-related lower urinary tract symptoms after surgery.
Our study revealed that the operation time was the only risk factor for postoperative febrile UTI. We observed that a long operation time requires massive irrigation, and this can be one of the reasons for the high incidence of UTI, although we did not measure the volume of irrigation fluid. The irrigation flow rate and the irrigation volume were previously reported as independent risk factors for UTI after URS. The high pressure in the renal pelvis, and reflux systemic absorption of irrigation fluid containing bacteria or toxins have been proven to contribute to the incidence of febrile UTI after URS [2122232425]. If the stone size is large, the operation time could be longer, but the stone size was not significantly correlated with the incidence of febrile UTI in our study. We also postulate that the composition (hardness) of the stones, rather than their size might contribute to a longer operation time.
This study has several limitations. First, this was a retrospective study with a small sample size. Second, because our institute is a tertiary hospital for lithotripsy, several patients in this study had complicated conditions, such as large or impacted stones and a comorbid status, which could increase the risk of postoperative UTI. Third, we did not analyze the effects of irrigation pressure or volume as potential risk factors for postoperative febrile UTI, as the irrigation flow rate and renal pelvic pressure were not measured in our study because manual irrigation was used during URS and RIRS, which made measurement of irrigation flow pressure impossible. This is one of the major limitations of this study. However, we have used a ureteral access sheath with a wide diameter (12/14-Fr) for RIRS during the study period in an effort to reduce irrigation flow pressure. Fourth, stone analysis was not routinely performed in this study, because dusting only without stone retrieval was performed in many cases. Among the cases in which stone analysis was performed, uric acid stone was the most common. Finally, this study was conducted in a single center, and as the resistance of pathogens to antibiotics can be different at each center, a further multi-center based study will be needed.
Despite adequate prophylactic antibiotics, postoperative febrile UTI after URS developed in 14.1% of patients at our institution. Although not all causative pathogens were identified, P. aeruginosa was the most frequently reported pathogen, which was isolated in more than half of the patients with a febrile UTI. In addition, most of the identified microorganisms were resistant to fluoroquinolones, which are routinely used as prophylactic antibiotics. Therefore, when a postoperative UTI is not well-controlled using prophylactic antibiotics, more potent antibiotics should be considered, even before the confirmation of pathogens. Moreover, to prevent postoperative UTI, it will be necessary to try to reduce the operation time.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (NRF-2015R1C1A1A01053009), (2016R1C1B1011180). Additional funding was provided by the Ministry of Education (2015R1D1A3A03020378) and the Ministry of Science, ICT & Future Planning (NRF-2014M3A9D3034164).
References
2. Takagi T, Go T, Takayasu H, Aso Y. Fiberoptic pyeloureteroscope. Surgery. 1971; 70:661–663. PMID: 5120887.
3. Bush IM, Goldberg E, Javadpour N, Chakrobortty H, Morelli F. Ureteroscopy and renoscopy: a preliminary report. Chic Med Sch Q. 1970; 30:46–49. PMID: 5317579.
4. Aboumarzouk OM, Monga M, Kata SG, Traxer O, Somani BK. Flexible ureteroscopy and laser lithotripsy for stones >2 cm: a systematic review and meta-analysis. J Endourol. 2012; 26:1257–1263. PMID: 22642568.
5. Stav K, Cooper A, Zisman A, Leibovici D, Lindner A, Siegel YI. Retrograde intrarenal lithotripsy outcome after failure of shock wave lithotripsy. J Urol. 2003; 170:2198–2201. PMID: 14634378.

6. Fabrizio MD, Behari A, Bagley DH. Ureteroscopic management of intrarenal calculi. J Urol. 1998; 159:1139–1143. PMID: 9507817.

7. Volkin D, Shah O. Complications of ureteroscopy for stone disease. Minerva Urol Nefrol. 2016; 68:570–585. PMID: 27441595.
8. Geavlete P, Georgescu D, Niţă G, Mirciulescu V, Cauni V. Complications of 2735 retrograde semirigid ureteroscopy procedures: a single-center experience. J Endourol. 2006; 20:179–185. PMID: 16548724.

9. Skolarikos A, Mitsogiannis H, Deliveliotis C. Indications, prediction of success and methods to improve outcome of shock wave lithotripsy of renal and upper ureteral calculi. Arch Ital Urol Androl. 2010; 82:56–63. PMID: 20593724.
10. Breda A, Ogunyemi O, Leppert JT, Schulam PG. Flexible ureteroscopy and laser lithotripsy for multiple unilateral intrarenal stones. Eur Urol. 2009; 55:1190–1196. PMID: 18571315.

11. Cindolo L, Castellan P, Scoffone CM, Cracco CM, Celia A, Paccaduscio A, et al. Mortality and flexible ureteroscopy: analysis of six cases. World J Urol. 2016; 34:305–310. PMID: 26210344.

12. Wolf JS, Bennett CJ, Dmochowski RR, Hollenbeck BK, Pearle MS, Schaeffer AJ. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol. 2008; 179:1379–1390. PMID: 18280509.

13. Knopf HJ, Graff HJ, Schulze H. Perioperative antibiotic prophylaxis in ureteroscopic stone removal. Eur Urol. 2003; 44:115–118. PMID: 12814685.

14. Mitsuzuka K, Nakano O, Takahashi N, Satoh M. Identification of factors associated with postoperative febrile urinary tract infection after ureteroscopy for urinary stones. Urolithiasis. 2016; 44:257–262. PMID: 26321205.

15. Sohn DW, Kim SW, Hong CG, Yoon BI, Ha US, Cho YH. Risk factors of infectious complication after ureteroscopic procedures of the upper urinary tract. J Infect Chemother. 2013; 19:1102–1108. PMID: 23783396.

16. Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol. 2013; 34:1–14. PMID: 23221186.

17. Kang CI, Kim J, Park DW, Kim BN, Ha US, Lee SJ, et al. Clinical practice guidelines for the antibiotic treatment of community-acquired urinary tract infections. Infect Chemother. 2018; 50:67–100. PMID: 29637759.

18. Kim ME, Ha US, Cho YH. Prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in female outpatients in South Korea: a multicentre study in 2006. Int J Antimicrob Agents. 2008; 31(Suppl 1):S15–S18. PMID: 18096373.

19. Polk RE, Johnson CK, McClish D, Wenzel RP, Edmond MB. Predicting hospital rates of fluoroquinolone-resistant Pseudomonas aeruginosa from fluoroquinolone use in US hospitals and their surrounding communities. Clin Infect Dis. 2004; 39:497–503. PMID: 15356812.

20. Grabe M, Botto H, Cek M, Tenke P, Wagenlehner FM, Naber KG, et al. Preoperative assessment of the patient and risk factors for infectious complications and tentative classification of surgical field contamination of urological procedures. World J Urol. 2012; 30:39–50. PMID: 21779836.

21. Tuzel E, Aktepe OC, Akdogan B. Prospective comparative study of two protocols of antibiotic prophylaxis in percutaneous nephrolithotomy. J Endourol. 2013; 27:172–176. PMID: 22908891.

22. Singla M, Srivastava A, Kapoor R, Gupta N, Ansari MS, Dubey D, et al. Aggressive approach to staghorn calculi-safety and efficacy of multiple tracts percutaneous nephrolithotomy. Urology. 2008; 71:1039–1042. PMID: 18279934.

23. Osther PJS. Risks of flexible ureterorenoscopy: pathophysiology and prevention. Urolithiasis. 2018; 46:59–67. PMID: 29151117.

24. Lildal SK, Andreassen KH, Christiansen FE, Jung H, Pedersen MR, Osther PJ. Pharmacological relaxation of the ureter when using ureteral access sheaths during ureterorenoscopy: a randomized feasibility study in a porcine model. Adv Urol. 2016; 10. 20. [Epub]. DOI: 10.1155/2016/8064648.

25. Osther PJ, Pedersen KV, Lildal SK, Pless MS, Andreassen KH, Osther SS, et al. Pathophysiological aspects of ureterorenoscopic management of upper urinary tract calculi. Curr Opin Urol. 2016; 26:63–69. PMID: 26555686.

Table 1
Baseline characteristics of patients
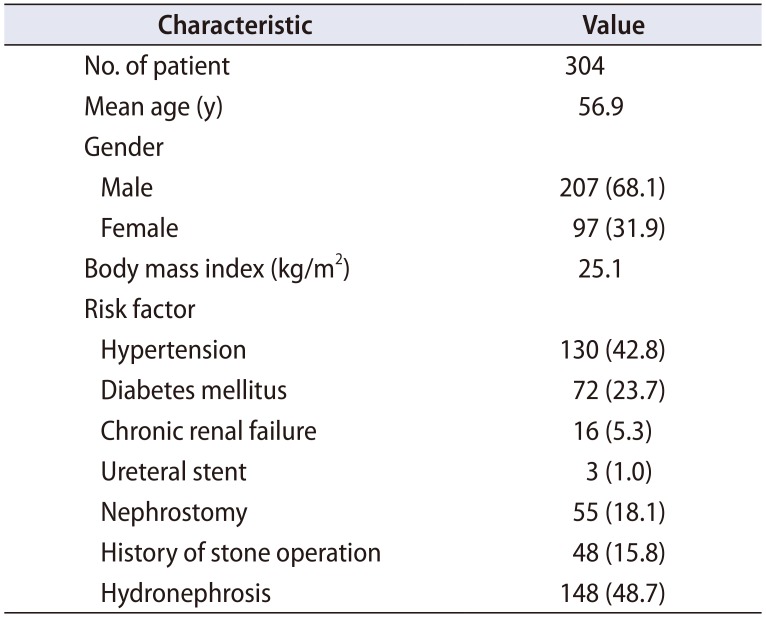
Table 2
Univariate and multivariate logistic regression analyses of febrile UTIs after ureteroscopic lithotripsy




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download