Abstract
Purpose
RENAL nephrometry score (RNS) was devised for deciding the approach for renal tumors. It is increasingly used in predicting perioperative outcomes with variable results. The actual difficulty encountered during surgery depends on a number of other variables. The main purpose of this prospective study was to identify these variables which are not addressed by current RNS.
Materials and Methods
Forty-nine patients undergoing robotic nephron sparing surgery from January 2015 onward were included. RNS was calculated from the imaging. Operating surgeon rated each surgery on a Likert scale of 0–4 after the completion of the procedure. The questionnaire was pre-validated in 5 cases before administration. The correlation between the surgeon rating and RNS with perioperative parameters and trifecta outcomes were calculated.
Results
Forty-seven percent surgeries were rated easy, and 53.0% were rated as difficult. Surgeries for hilar, posterior location and presence of supernumerary vessels were found to be the a cause of difficulty. Trifecta outcomes were achieved in 37/49 patients (75.5%). The mean rating was 2.580±0.900 in trifecta negative patients while it was 1.410±0.832 in trifecta positive patient (p<0.0001). Surgeon's rating correlated positively with trifecta outcomes (likelihood ratio=15.75, p=0.006).
Conclusions
The RNS remained a useful tool for determining renal tumor complexity. The intraoperative difficulty faced by the surgeon can be rated which can better predict perioperative trifecta outcomes. A useful predicting tool can be developed using the two parameters (RNS and surgeon rated difficulty).
RENAL nephrometry score (RNS) was initially devised for decision making regarding the approach for renal tumors. It was implicated that patients with high RNS (RNS>10) were more likely to undergo radical nephrectomy instead of nephron sparing surgery (NSS) [1]. However, with increasing surgeon's experience, even difficult tumors are being treated by NSS [23]. The robotic platform has also contributed to the same. The earliest premise of low (4–6), intermediate (7–9), and high (10–12) complexity seems to be fading with recent evidence [34]. More data is accumulating regarding NSS in high complexity tumors.
Combined perioperative outcomes as a measure of quality control and uniformity are being introduced in oncologic surgeries. The concept of ‘trifecta’ outcomes of robotic or laparoscopic NSS was first introduced by Hung et al. [5]. Since then a number of definitions have been described. Categorization of such combined outcomes in a binary fashion gives objective parameters for comparison across various studies. However, there is lack of uniformity in the definition of these outcomes; thereby caution should be exercised during their interpretation. Trifecta outcomes originally included negative tumor margins, functional preservation of kidney and no urologic complications [5]. It was stated that trifecta achievement should be a routine goal during NSS surgery [56]. In a recent multi institutional retrospective study by Zargar et al. [7], a relatively objective definition of trifecta was stated as negative surgical margin, minimal postoperative complications and warm ischemia time (WIT) of ≤25 minutes.
On similar lines, we defined trifecta outcomes for this study as the achievement of negative surgical margins, WIT <30 minutes and minimal complications (Clavien Dindo grade 1 and 2). We try to keep WIT to less than 30 minutes as the upper limit for NSS with recent literature backing up the same value [89].
The literature regarding RNS and its correlation to these perioperative outcomes has been controversial [1011]. Acar et al. [12] have found that there was no statistically significant difference in RENAL, PADUA, and C-index between the trifecta positive and negative groups. A plausible explanation for this poor correlation is that these scoring systems were primarily devised for predicting tumor complexity and anticipatory difficulty during NSS rather than the surgical outcome.
However, the perioperative outcomes depend on some factors apart from the RNS. The actual difficulty encountered during surgery depends on a number of factors, such as surgeon's experience, hilar anatomy, vascular anomalies, and tumor characteristics [13]. Postoperative complications which are a part of trifecta outcomes also depend on patient's age and comorbidities [14]. Thus, these scoring systems alone may not be reflective of perioperative outcomes. This was our premise for hypothesizing that an experienced surgeon's rating regarding the actual difficulty encountered during surgery is likely to correlate with intraoperative variables, such as operative time (OT), estimated blood loss (EBL), and WIT and also likely to correlate well with perioperative complications and trifecta outcomes. Identifying these variables and future incorporation of the same would improve the preoperative prediction about perioperative and trifecta outcomes.
Forty-nine consecutive patients, undergoing robot-assisted nephron-sparing surgery (RANSS) from January 2015 were included in the study. An informed consent was obtained from all the patients. The study was approved by institute ethics committee (approval number: INT/IEC/2015/386). The demographic details were noted. Imaging viz. computed tomography scans were viewed by an urologist and RNS was calculated according to method described by Kutikov and Uzzo [1].
A surgeon perspective questionnaire was filled by the operating surgeon for these 49 robotic cases immediately after the completion of procedure (to reduce the recall bias). The two operating surgeons have equivalent experience and have achieved the learning curve for RANSS. The surgical procedure was rated on a Likert scale of 0–4. Zero and 1 rating being ‘very easy’ and ‘easy’, respectively. In such cases further questions were not answered by the surgeon. However, if the rating was 2 (slightly difficult), 3 (difficult), or 4 (very difficult), the surgeon further answered questions regarding reasons for difficulty encountered, incorporated in an objective questionnaire (Supplementary Table). The correlation between the rating, RNS, perioperative parameters and trifecta outcomes was calculated.
Statistical analyses were performed using IBM SPSS Statistics ver. 22.0 software (IBM Co., Armonk, NY, USA) and Microsoft Excel 2007 (Microsoft, Redmond, WA, USA). Discrete categorical data was represented in the form of either a number or a percentage. Continuous data, assumed to be normally distributed, was written in the form of its mean and standard deviation, when it was skewed it was written in the form of its median and interquartile range, as per the requirement. The normality of quantitative data was checked by measures of Kolmogorov-Smirnov tests of normality. For skewed data Kruskal-Wallis test followed by Mann-Whitney test for two groups was applied. To compare the two groups for normally distributed data, Student t-test was applied. Proportions were compared using chi-square or Fisher's exact test, depending on their applicability for two groups. Spearman or Pearson correlation coefficients were calculated to see relationship of different variables. For time related variables of skewed data Wilcoxon Signed rank test was applied while for normally distributed data paired t-test was carried out. All the statistical tests were two-sided and were performed at a significance level of p<0.05.
Mean age of the patients was 50.27±13.22 years. Thirty-one patients were male. Mean body mass index was 24.12±3.97 kg/m2. Median follow-up of the patients was 8 months and mean follow-up was 8.43±3.66 months. Mean tumor size was 5.75±2.00 cm. Seventy-eight percent of the patients had malignant histopathology. Amongst them, most common was clear cell carcinoma (67.3%). Majority (42.9%) tumors were T1b. Mean and median RNS was 7.51±1.69 and 7 (6–9), respectively. Most common score for the subcomponents of the RNS was 2. Majority (46.9%) of tumors belong to intermediate group. Eleven tumors (22.5%) belonged to high RNS group while 15 tumors (30.6%) were of low RNS. Majority of the tumors were located anteriorly.
The preoperative estimated glomerular filtration rate (eGFR), postoperative eGFR and eGFR at last follow-up was 90.1±30.4 mL/min, 86.5±29.7 mL/min, and 90.00±29.80 mL/min, respectively. The difference between the last eGFR and preoperative eGFR was not statistically significant (p=0.639). Mean OT and WIT was 165.31±65.64 minutes and 24.50±12.87 minutes, respectively. Mean and median EBL was 206.6±178.6 mL and 150 mL (100–225 mL), respectively. Pelvicalyceal system was entered in 21 patients. Supernumerary arteries were present in 9 patients and supernumerary veins were present in 7 patients. Complications were graded as per Clavien Dindo complication grading. Majority (28.6%) of the complications were grade 1, followed by grade 2 (12.2%) and grade 3 (2.0%). Most common complication was postoperative fever. Mean length of hospital stay was 5.76±2.00 days.
As per operating surgeon's rating, four surgeries (8.2%) were rated as very easy, 19 (38.8%) were rated as easy, 16 (32.7%) were rated as slightly difficult, 8 (16.3%) rated as difficult, and 2 (4.1%) as very difficult. Twenty-six surgeries which were rated as more than 1 were elaborately studied for the cause of difficulty. More than 1 reason was cited in most cases. Surgeries done for tumors located in hilar and posterior location were incriminated as difficult. Interestingly, in two of the cases, anterior location of tumor was mentioned as cause of difficulty due to decreased space between the abdominal wall and the tumor. Apart from high RNS, hilar anatomy including presence of supernumerary vessels was also found to be cause of difficulty in many patients (Table 1). Two patients had previous surgeries included 1 patient of von Hippel-Lindau disease (Fig. 1) and other was a renal transplant patient with tumor in transplanted kidney.
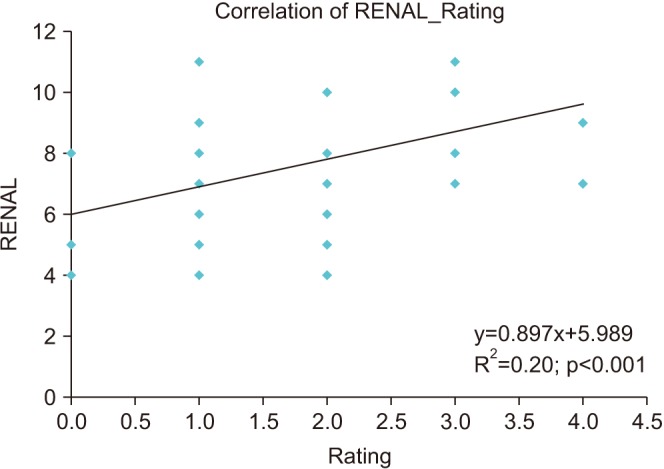
Trifecta outcomes were achieved in 37 patients (75.5%). Table 2 depicts the correlation of various variables with surgeon's rating of difficulty level. The rating given by surgeon correlated positively with RNS (Fig. 2), OT, WIT, EBL and tumor size (Table 2). While RNS correlated with WIT, EBL, and tumor size and not with OT. It is imperative that the difficulty rating reflects all the above mentioned parameters. There was statistically significant difference in surgeon's rating in patients with trifecta positive patients (1.410±0.832) versus trifecta negative patients (2.580±0.900) (p<0.0001). No such statistically significant difference was seen in RNS between the two groups (p=0.154). Surgeon's rating correlated significantly with trifecta outcomes (Pearson chi-square=14.47, p=0.006). The rating showed positive correlation with complications (graded as per Clavien Dindo grading) (Spearman Rho=0.437, p=0.002). Receiver operating characteristics (ROC) curves were plotted for RNS, surgeon's rating, RNS+Surgeon's rating and RNS in combination with other variables with respect to trifecta outcomes (Fig. 3). Other variables included previous surgery, hilar anatomy, large amount of perinephric fat and location of tumor. Each factor was given a score of one by the surgeon if present. Area under curve (AUC) of the plots was as follows: RNS=0.636, Rating=0.821, RNS+Rating=0.729, and RNS+other variables=0.747.
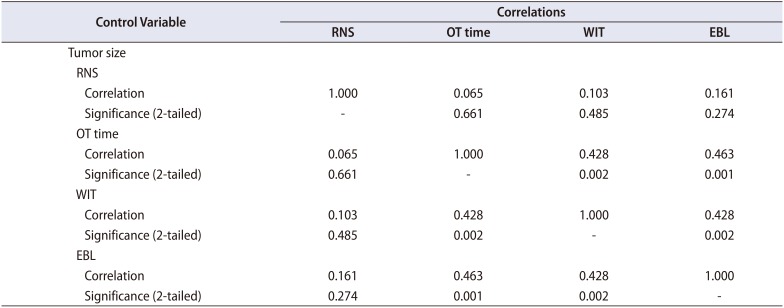
Okhunov et al. [10] conducted a study to establish reliability of threescoring systems (RENAL, PADUA, and C-index) and assessed relationships between these scoring systems and perioperative and postoperative variables. They found that there were no significant associations between any of the three scoring systems assessed and the occurrence of complications, OT, or EBL. All the scores were associated with WIT, percent change in creatinine level, and tumor size. In our series the RNS had good correlation with WIT, EBL, and tumor size. On the contrary, Yeon et al. [15] have found that RENAL and PADUA score do not predict perioperative outcomes viz. complications, WIT, and EBL. Thus, the issue of predictive value for perioperative outcomes of already available scoring systems remains controversial. Similarly, when we adjusted for tumor size to avoid problem of multicollinearity the RNS did not correlate with any of the perioperative variables implying tumor size becomes a major predictor of trifecta outcomes (Table 3).
In our study, the surgeon's rating correlated positively with OT, WIT, EBL, and tumor size. Thus, implying almost all the important intraoperative parameters are reflected by surgeon's rating. The rating correlated well with RNS as well (p=0.001) implying that RNS can fairly predict intraoperative difficulty encountered by surgeon preoperatively. The surgeon's rating had good correlation with trifecta outcomes. A combination of tumor complexity (RNS) and intraoperative difficulty is also likely correlate with trifecta outcomes better than the scoring systems alone. When we look at the ROC curves it can be seen that a combination of RNS with other variables and RNS with surgeon's rating has similar AUC (Fig. 3). The AUC for RNS was 0.636, while the AUC for RNS+rating and RNS+other variables was 0.729 and 0.747, respectively. Thus, these two AUC increase the predictability over RNS alone for trifecta outcomes. RNS +rating has a subjective assessment, however the variables identified, when added to the RNS scoring maintained the improvement in predictability. This implies that addition of these variables to the RNS may provide for a fair predictive tool for trifecta outcomes in future.
In a recent study by Sharma et al. [16], single surgeon intuition to choose a particular approach for NSS versus prediction by RNS and Mayo adhesive probability (MAP) score was calculated in 119 patients. Patients undergoing open partial nephrectomy (OPN) had higher median nephrometry scores compared to Robot assisted transperitoneal NSS and Robot assisted retroperitoneal NSS patients (8 vs. 7 vs. 7, respectively; p=0.03), but MAP scores were not different among all three groups (p=0.36). RNS was found to be predictive of OPN. A score of 6.5 had the highest sensitivity and specificity (76% and 42%, respectively) for predicting OPN. They concluded RNS was associated with surgical approach intuitively chosen by an experienced surgeon, but the presence of adherent perinephric fat did not correlate with decision-making. This study strengthens our premise that an experienced surgeon's intuition regarding surgery in association with validated scoring system will be a better predictor of trifecta outcomes.
The surgeon's rating in our study is a postoperative variable in contrast to the preoperatively used RNS. The surgeon's score is likely a sum of all the factors which are apart from tumor complexity which include adhesions due to previous surgeries, presence of supernumerary vessels, increased perinephric fat and even use of defective instrument or inexperience of the assistant. This score at the end of surgery can be an added asset in prediction of trifecta outcomes or any such combined outcomes used by institutions for their quality control and patient's counselling. The RNS primarily predicts the tumor complexity but at times high RNS tumors are operated easily and vice versa. The prediction of trifecta will be better depending upon the intraoperative detailing of the surgical procedure.
The premise of RNS as the predictor of feasibility of surgery based on initial categorization into low, intermediate and high is blurring. In analysis of 79 patients undergoing NSS at Post Graduate Institute of Medical Education and Research we found no difference between the outcomes in low and intermediate group patients [17]. An actual quantifiable variable in form of surgeon's rating can strongly predict the trifecta outcomes and can be used alone or in a nomogram to predict perioperative outcomes. Such variables in combination with scoring system will be of utility in perioperative counselling of the patients. They will also help in alerting the managing team regarding anticipated postoperative complications in case where surgeon's rating was of higher difficulty level.
The study has the following limitations: a small sample size, the subjective nature of questionnaire, and the inherent bias of surgeon's rating when done postoperatively. However, we deliberately used post procedure rating instead of a pre procedural intuitive rating for this study as a measure of difficulty encountered during surgery. Rating given immediately at the end of procedure removed recall bias and very well quantified the intraoperative events a single variable. It also helped us in documenting the important determinants of perioperative outcomes, such as posterior location of tumor, hilar anatomy, large perinephric fat, and previous surgery which are not quantified in these scoring systems. A comparison with a preoperative surgeon's score is underway at Post Graduate Institute of Medical Education and Research. An external validation of the questionnaire is needed by further studies.
Surgeon's rating correlated with individual perioperative outcomes (OT, WIT, and EBL) and combined trifecta outcomes. Intraoperative difficulty rated by surgeon has a good correlation with trifecta outcomes. The scoring system remains a useful tool for determining renal tumor complexity. However, perioperative combined outcomes were not associated with RNS. The current study concludes that surgeon's rating is a valuable tool in predicting perioperative outcomes, helping in patient counselling, treatment planning, and postoperative management after nephron sparing surgery. Addition of new parameters, such as posterior location, hilar anatomy, and prior surgery may improve predictive value of RNS for trifecta outcomes However, a larger study is needed to confirm and validate these findings.
References
1. Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009; 182:844–853. PMID: 19616235.

2. Gupta GN, Boris R, Chung P, Linehan WM, Pinto PA, Bratslavsky G. Robot-assisted laparoscopic partial nephrectomy for tumors greater than 4 cm and high nephrometry score: feasibility, renal functional, and oncological outcomes with minimum 1 year follow-up. Urol Oncol. 2013; 31:51–56. PMID: 21292511.

3. Abdel Raheem A, Alatawi A, Kim DK, Sheikh A, Alabdulaali I, Han WK, et al. Outcomes of high-complexity renal tumours with a Preoperative Aspects and Dimensions Used for an Anatomical (PADUA) score of ≥10 after robot-assisted partial nephrectomy with a median 46.5-month follow-up: a tertiary centre experience. BJU Int. 2016; 118:770–778. PMID: 27102977.

4. Kim DK, Kim LH, Raheem AA, Shin TY, Alabdulaali I, Yoon YE, et al. Comparison of trifecta and pentafecta outcomes between T1a and T1b renal masses following robot-assisted partial nephrectomy (RAPN) with minimum one year follow up: can RAPN for T1b renal masses be feasible? PLoS One. 2016; 11:e0151738. PMID: 26987069.

5. Hung AJ, Cai J, Simmons MN, Gill IS. “Trifecta” in partial nephrectomy. J Urol. 2013; 189:36–42. PMID: 23164381.

7. Zargar H, Allaf ME, Bhayani S, Stifelman M, Rogers C, Ball MW, et al. Trifecta and optimal perioperative outcomes of robotic and laparoscopic partial nephrectomy in surgical treatment of small renal masses: a multi-institutional study. BJU Int. 2015; 116:407–414. PMID: 25220543.
8. Parekh DJ, Weinberg JM, Ercole B, Torkko KC, Hilton W, Bennett M, et al. Tolerance of the human kidney to isolated controlled ischemia. J Am Soc Nephrol. 2013; 24:506–517. PMID: 23411786.

9. Mir MC, Pavan N, Parekh DJ. Current paradigm for ischemia in kidney surgery. J Urol. 2016; 195:1655–1663. PMID: 26804756.

10. Okhunov Z, Rais-Bahrami S, George AK, Waingankar N, Duty B, Montag S, et al. The comparison of three renal tumor scoring systems: C-Index, P.A.D.U.A., and R.E.N.A.L. nephrometry scores. J Endourol. 2011; 25:1921–1924. PMID: 21905850.

11. Roushias S, Vasdev N, Ganai B, Mafeld S, Rix D, Thomas D, et al. Can the R.e.N.a.L nephrometry score preoperatively predict postoperative clinical outcomes in patients undergoing open and laparoscopic partial nephrectomy? Curr Urol. 2013; 7:90–97. PMID: 24917765.

12. Acar Ö, Işık EÖ, Mut T, Sağlıcan Y, Onay A, Vural M, et al. Comparison of the trifecta outcomes of robotic and open nephron-sparing surgeries performed in the robotic era of a single institution. Springerplus. 2015; 4:472. PMID: 26361573.

13. Qi J, Yu Y, Huang T, Bai Q, Kang J, Liang J, et al. Predictors of postoperative renal functional damage after nephron-sparing surgery. World J Surg Oncol. 2013; 11:216. PMID: 23987305.

14. Hew MN, Baseskioglu B, Barwari K, Axwijk PH, Can C, Horenblas S, et al. Critical appraisal of the PADUA classification and assessment of the R.E.N.A.L. nephrometry score in patients undergoing partial nephrectomy. J Urol. 2011; 186:42–46. PMID: 21571340.

15. Yeon JS, Son SJ, Lee YJ, Cha WH, Choi WS, Chung JW, et al. The nephrometry score: is it effective for predicting perioperative outcome during robot-assisted partial nephrectomy? Korean J Urol. 2014; 55:254–259. PMID: 24741414.

16. Sharma P, McCormick BZ, Zargar-Shoshtari K, Sexton WJ. Is surgeon intuition equivalent to models of operative complexity in determining the surgical approach for nephron sparing surgery? Indian J Urol. 2016; 32:124–131. PMID: 27127355.

17. Bora GS, Mavuduru RM, Sharma AP, Devana SK, Kakkar N, Lal A, et al. Initial experience of robotic nephron sparing surgery in cases of high renal nephrometry scores. Indian J Urol. 2017; 33:230–235. PMID: 28717275.

SUPPLEMENTARY MATERIAL
Scan this QR code to see the supplementary material, or visit https://www.icurology.org/src/sm/icurology-59-305-s001.pdf.

Fig. 1
Computed tomography scans showing tumor in the right kidney of a patient with von Hippel-Lindau disease. Note the ventriculo peritoneal shunt in situ (arrow).

Fig. 3
Receiver operating characteristics (ROC) curves for predictors of trifecta; (A) RENAL nephrometry score (RNS), (B) surgeon's rating, (C) RNS+rating and RNS+other variables.

Table 1
Reason for difficulty encountered as noted in surgeon perspective questionnaire
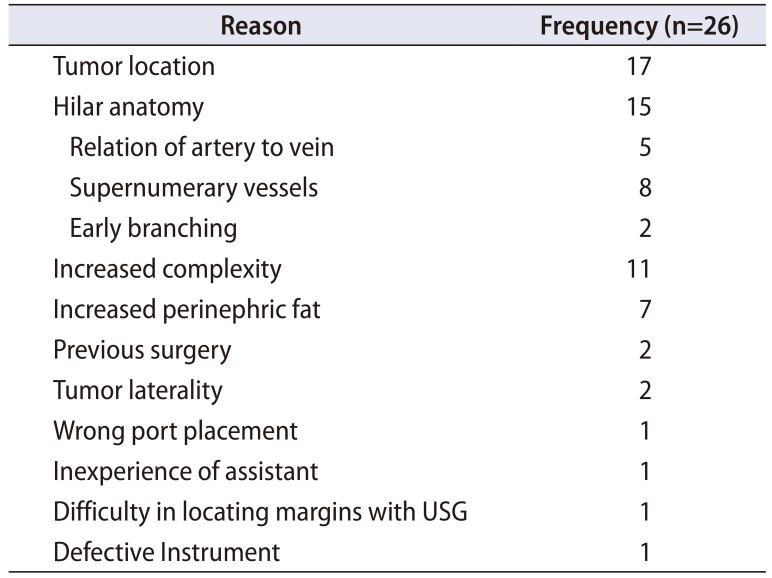
Table 2
Correlation of RNS with perioperative parameters
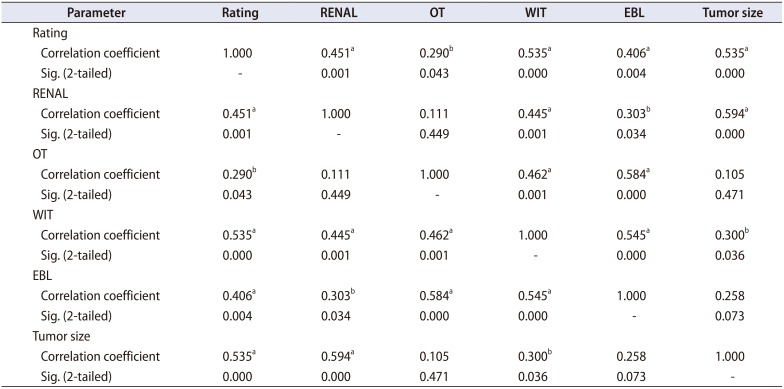
Table 3
Partial correlation of RNS with OT, WIT, and EBL after controlling for tumor size
We have reviewed with great enthusiasm the article entitled “Predicting trifecta outcomes after robot-assisted nephron-sparing surgery: Beyond the nephrometry score” that will publish in Investigative and Clinical Urology. We congratulate the authors for this informative and comprehensive review. There are few comments.
In the article, the main purpose of this prospective study was to identify the actual difficulty encountered during surgery depends on a number of other variables which are not addressed by current RENAL nephrometry score (RNS).
In a prospective analysis, the authors evaluated the correlation between the surgeon difficulty rating (on a Likert scale of 0–4 after the completion of the procedure) and RNS with perioperative parameters and trifecta outcomes were calculated.
Including 49 patients undergoing robot-assisted nephron-sparing surgery (RANSS) in a single institution from January 2015 by two experienced in RANSS surgeons. The mean and median of RNS was 7.51±1.69 and 7 (6–9), respectively. Most common score for the subcomponents of the RNS was 2. Majority of the tumors were located anteriorly. The operating surgeon's rating, 4 surgeries (8.2%) were rated as very easy, 19 (38.8%) were rated as easy, 16 (32.7%) were rated as slightly difficult, 8 (16.3%) rated as difficult, and 2 (4.1%) as very difficult. Trifecta outcomes were achieved in 37 patients (75.5%).
Prospective analysis outcomes. The rating given by surgeon correlated positively with RNS, operative time (OT), warm ischemia time (WIT), estimated blood loss (EBL) and tumor size. While RNS correlated with WIT, EBL, and tumor size but not with OT. There was statistically significant difference in surgeon's rating in patients with trifecta positive patients group (1.410±0.832) versus trifecta negative patients group (2.580±0.900) (p<0.0001). No statistically significant difference was seen in RNS between the two groups (p=0.154).
In this study, we found that the surgeon's rating correlated well with RNS (p=0.001) implying that RNS can fairly predict intraoperative difficulty encountered by surgeon preoperatively. The surgeon's score is likely a sum of all the factors which are apart from tumor complexity which include adhesions due to previous surgeries, presence of supernumerary vessels, increased perinephric fat and even use of defective instrument or inexperience of the assistant. The surgeon's rating had good correlation with trifecta outcomes. A combination of tumor complexity (RNS) and intraoperative difficulty are also likely correlate with trifecta outcomes better than the scoring systems alone.
Subsequent studies might be comparable of this surgeon's rating score in difference of hilar control [12], more number of patients and multi-center or systematic review to reduce confounding factors [3]. An external validation of the questionnaire is needed to reduce the subjective nature of questionnaire and the inherent bias of surgeon's rating when done postoperatively.
We believe that in experienced surgeon could operate robotic nephron-sparing surgery in even high RNS with achievement the concept of trifecta [45]. And we also agree that the surgeon's rating is a valuable tool in predicting perioperative outcomes, helping in patient counselling, treatment planning and postoperative management after nephron-sparing surgery.
References
1. Cacciamani GE, Medina LG, Gill TS, Mendelsohn A, Husain F, Bhardwaj L, et al. Impact of renal hilar control on outcomes of robotic partial nephrectomy: systematic review and cumulative meta-analysis. Eur Urol Focus. 2018; 2. 05. [Epub]. DOI: 10.1016/j.euf.2018.01.012.

2. Abdel Raheem A, Santok GD, Kim LHC, Chang KD, Lum TGH, Yoon YE, et al. Off-clamp robot-assisted partial nephrectomy: how far shall we proceed? J Laparoendosc Adv Surg Tech A. 2018; 28:579–585. PMID: 29048977.

3. Volpe A, Blute ML, Ficarra V, Gill IS, Kutikov A, Porpiglia F, et al. Renal ischemia and function after partial nephrectomy: a collaborative review of the literature. Eur Urol. 2015; 68:61–74. PMID: 25703575.

4. Hung AJ, Cai J, Simmons MN, Gill IS. “Trifecta” in partial nephrectomy. J Urol. 2013; 189:36–42. PMID: 23164381.

5. Zargar H, Allaf ME, Bhayani S, Stifelman M, Rogers C, Ball MW, et al. Trifecta and optimal perioperative outcomes of robotic and laparoscopic partial nephrectomy in surgical treatment of small renal masses: a multi-institutional study. BJU Int. 2015; 116:407–414. PMID: 25220543.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download