This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
The purpose of this study was to evaluate the efficacy of treatment in patients with non-bothering nocturia.
Materials and Methods
In this prospective multicenter study, patients who visited hospitals for treatment of voiding symptoms were enrolled. Inclusion criteria were: 1) men >45 years, and 2) nocturia ≥2 confirmed by a three-day voiding diary. Subjects were divided into non-bothering and bothering groups based on International Consultation on Incontinence Questionnaire Nocturia (ICIQ-N) question 2b. Changes in voiding symptoms, frequency of nocturia, and bothersomeness were evaluated with international prostate symptom score (IPSS), ICIQ-N, and three-day voiding diary at 4 and 12 weeks after treatment.
Results
A total of 48 patients in the non-bothering nocturia group and 50 patients in the bothering nocturia group who completed the 12-week treatment were analyzed. The total IPSS was decreased by 5.8 in the non-bothering group and 5.2 in the bothering group. There was no significant difference in decrease of IPSS between the two groups. Both groups showed significant reduction in discomfort of nocturia. The ICIQ-N 2b score decreased from 3.9 to 2.7 (p=0.01) in the non-bothering group and from 6.9 to 4.6 (p=0.02) in the bothering group. The number of nocturia episodes was significantly decreased in both groups.
Conclusions
Regardless of discomfort associated with nocturia, both groups showed significant improvement in nocturia-related discomfort and voiding symptoms. These results suggest that patients with nocturia who were unaware of its discomfort benefited from treatment.
Go to :

Keywords: Lower urinary tract symptoms, Nocturia, Prostatic hyperplasia, Therapeutics
INTRODUCTION
Nocturia is one of the most bothersome lower urnary tract symptoms (LUTS) [
1]. Nocturia can be caused by various conditions. It might be due to several urologic diseases (such as benign prostate hyperplasia and overctive bladder) and other diseases such as diabetes, heart failure, and sleep disorder [
2345].
When nocturia causes discomfort to patients, it requires treatment. Nocturia can cause day-time sleepiness, reduce daily life activity, and increase the risk of falls [
6789]. Therefore, nocturia-related symptoms need to be treated to improve the quality of life of patients.
However, there is no consensus about the need for treatment of nocturia that does not bother patients. No study has reported characteristics or prognosis of nocturia that does not bother patients. Therefore, the objective of this study was to evaluate the efficacy of nocturia treatment on changing perceptions of discomfort in patients with non-bothering nocturia.
Go to :

MATERIALS AND METHODS
1. Subject
This prospective multicenter study was conducted at 12 sites in Korea. Patients who visited hospitals for treatment of voiding symptoms were enrolled as subjects. Inclusion criteria were: 1) men >45 years of age, 2) daily nocturia frequency of 2 or more confirmed with a three-day voiding diary, 3) voiding symptoms lasting ≥3 months, 4) total international prostate symptom score (IPSS) ≥8, and 5) prostate volume greater than 20 mL on transrectal ultrasonography. Because nocturia ≥2 times was significantly related to bothersomeness in a previous study, we did not include men with a single event of nocturia [
10].
Patients who met one of the following conditions were excluded from this study: insomnia or drug treatment for insomnia, nocturnal polyuria, acute urinary tract infection, use of an indwelling catheter or self-catheterization, intake of drugs including diuretics, alpha-blockers, five-alpha reductase inhibitor (5ARI), or anti-muscarinic use within three months of baseline, history of prostate or bladder cancer, and pelvic neuromuscular disease or surgery.
2. Ethics statement
The present study protocol was reviewed and approved by the Institutional Review Board of Konkuk University Hospital (KUH 2014-005) and each study center. Informed consent was submitted by all subjects when they were enrolled. And this study was conducted in accordance with the Declaration of Helsinki.
3. Study design
The flow-chart outlining the study design is provided in
Fig. 1. In addition to screening patients for history, they were surveyed using the IPSS questionnaire, International Consultation on Incontinence Questionnaire Nocturia (ICIQ-N) questionnaire, and a three-day voiding diary. Patients were subjected to a laboratory examination including urinalysis and prostate specific antigen (PSA), and evaluated with transrectal ultrasonography following nocturia treatment at baseline.
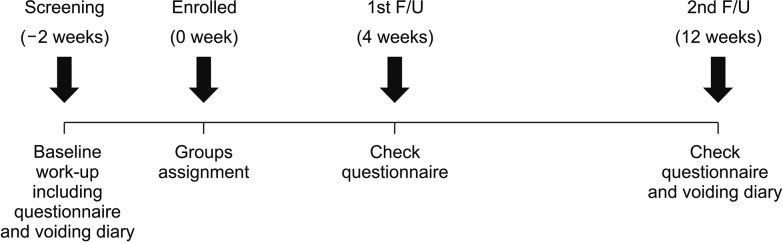 | Fig. 1The schematic showing study protocol. F/U: follow-up.
|
After screening, eligible subjects were divided into non-bothering and bothering groups according to their response to the following question on nocturia: “Do you feel bothersome when you wake up to void at night?” the patients who replied “Yes” were placed in the bothering group and those who replied “No” were included in the non-bothering group. Based on the judgment of the individual physician, each patient in either group received treatment with drugs such as alpha blocker, 5ARI, and desmopressin for 12 weeks.
Study visits were conducted during week 0 (visit 1, confirmation of eligibility criteria), week 4 (visit 2), and week 12 (visit 3, follow-up visit and at the end of the treatment). Changes in voiding symptoms, frequency of nocturia, and nocturia bothersomeness were evaluated with IPSS, ICIQ-N questionnaire, and three-day voiding diary at week 4 and week 12.
4. Efficacy outcome measures
Primary efficacy endpoint was change in nocturia bothersomeness from baseline to the final visit in both groups after 12 weeks of treatment. Degree of nocturia bothersomeness was evaluated using ICIQ-N questionnaire item 2b visual analogue scale (VAS) ranging from 0 to 10 points. Secondary efficacy endpoints were changes in nocturia episodes measured by 3-day voiding diary and changes in LUTS measured by IPSS.
A sample size of 62 in each group provided 80% power to compare the non-bothering group and the bothering group with a two-sided significance level of 5%. Sample size was calculated based on the assumption of a 10% decrease of VAS in the non-bothering group and 30% decrease of VAS in the bothering group. Considering a 30% dropout rate, the target number of subjects was set at 89 patients per group.
5. Statistical analysis
Statistical analyses were performed using PASW vers. 17.0 software (IBM Co., Armonk, NY, USA). Changes in nocturia bothersomeness VAS, American Urological Association Symptom Score, and the number of nocturia episodes were compared between the two groups using Student's t-tests and Mann-Whitney U-test. Categorical data were analyzed using chi-squared test. Reported p-value were two-sided. Statistical significance was set at p<0.05.
Go to :

RESULTS
A total of 142 patients were enrolled in this study between August 2013 and December 2015. The number of patients in the non-bothering group was 66 and those in the bothering group was 76. Eighteen patients in the non-bothering group and 26 patients in the bothering group dropped out, including 24 patients who stopped taking the medication due to insufficient efficacy (n=10), adverse effects including dry mouth and orthotopic hypotension (n=12), and other reasons (n=2), five patients who withdrew consent, and 15 patients who were lost to follow-up. Overall, 98 patients (48 in the non-bothering group and 50 in the bothering group) who completed the 12-week treatment were analyzed.
1. Demographic and clinical characteristics
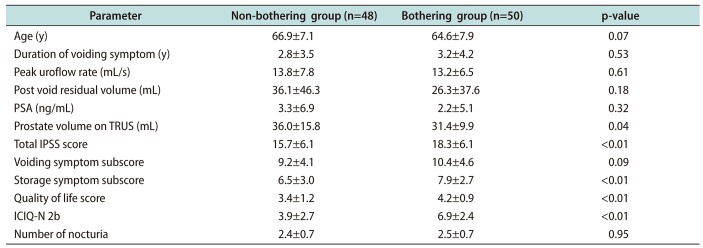
Patient demographics are summarized in
Table 1. There was no significant difference in age, symptom duration, peak flow rate, post void residual volume, and PSA. However, prostate volume in the non-bothering group was slightly but significantly larger than in the bothering group (36.0±15.8 mL
vs. 31.4±9.9 mL, p=0.04;
Table 1).
Table 1
Characteristics of subjects
|
Parameter |
Non-bothering group (n=48) |
Bothering group (n=50) |
p-value |
|
Age (y) |
66.9±7.1 |
64.6±7.9 |
0.07 |
|
Duration of voiding symptom (y) |
2.8±3.5 |
3.2±4.2 |
0.53 |
|
Peak uroflow rate (mL/s) |
13.8±7.8 |
13.2±6.5 |
0.61 |
|
Post void residual volume (mL) |
36.1±46.3 |
26.3±37.6 |
0.18 |
|
PSA (ng/mL) |
3.3±6.9 |
2.2±5.1 |
0.32 |
|
Prostate volume on TRUS (mL) |
36.0±15.8 |
31.4±9.9 |
0.04 |
|
Total IPSS score |
15.7±6.1 |
18.3±6.1 |
<0.01 |
|
Voiding symptom subscore |
9.2±4.1 |
10.4±4.6 |
0.09 |
|
Storage symptom subscore |
6.5±3.0 |
7.9±2.7 |
<0.01 |
|
Quality of life score |
3.4±1.2 |
4.2±0.9 |
<0.01 |
|
ICIQ-N 2b |
3.9±2.7 |
6.9±2.4 |
<0.01 |
|
Number of nocturia |
2.4±0.7 |
2.5±0.7 |
0.95 |

2. Baseline voiding symptom
1) International prostate symptom score
The bothering group showed a higher total IPSS (18.3±6.1
vs. 15.7±6.1, p<0.01), storage symptom subscore (7.9±2.7
vs. 6.5±3.0, p<0.01), and quality of life score (4.2±0.9
vs. 3.4±1.2, p<0.01) than the non-bothering group. There was no significant difference in voiding symptom subscore between the two groups (10.4±4.6
vs. 9.2±4.1, p=0.09) (
Table 1).
2) Nocturia-related symptoms
Bothersomeness evaluated by ICIQ-N 2b VAS was higher in the bothering group than in the non-bothering group (6.9±2.4
vs. 3.9±2.7, p<0.01). However, the number of nocturia episodes was not significantly different between the two groups (2.5±0.7
vs. 2.4±0.7, p=0.95) (
Table 1).
3. After 12 weeks of treatment
1) International prostate symptom score
Both groups showed significant improvement in LUTS (
Table 2). Total IPSS was decreased by 5.8 in the non-bothering group and 5.3 in the bothering group. The difference in the degree of decrease in IPSS between the two groups was not statistically significant (
Fig. 2A).
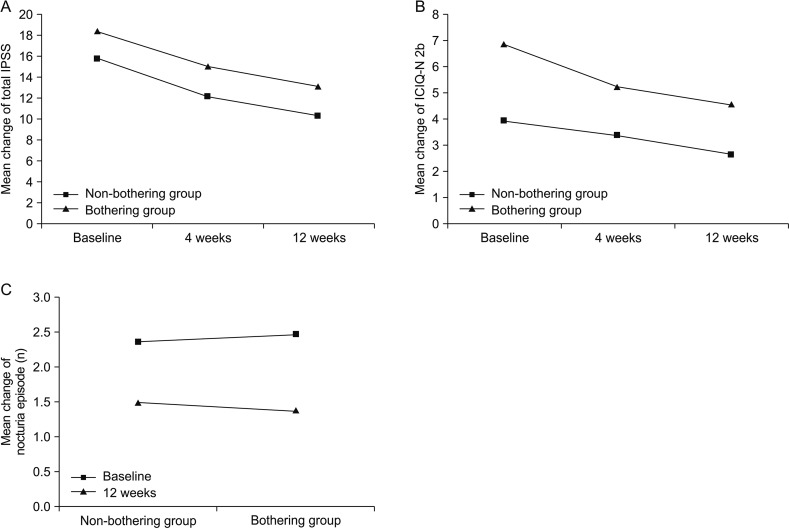 | Fig. 2Change of variables after 12-week treatment. (A) The change of total international prostate symptom score (IPSS). (B) The change of International Consultation on Incontinence Questionnaire Nocturia (ICIQ-N) 2b visual analogue scale. (C) The change of nocturia episode.
|
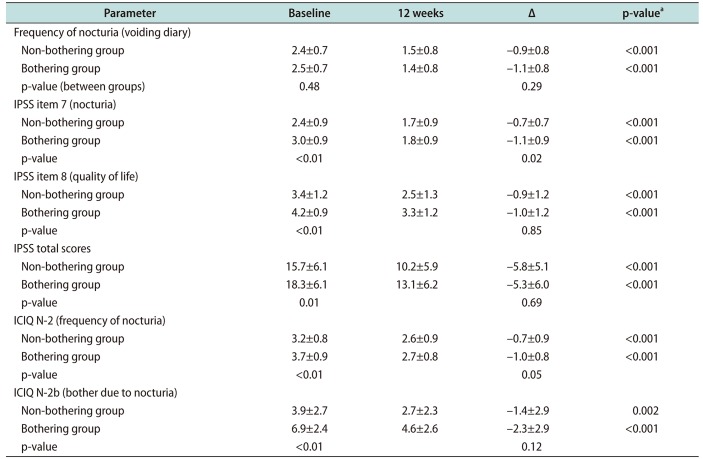
Table 2
Change of parameters related to lower urinary tract symptoms following treatment for 12 weeks
|
Parameter |
Baseline |
12 weeks |
Δ |
p-valuea
|
|
Frequency of nocturia (voiding diary) |
|
|
|
|
|
Non-bothering group |
2.4±0.7 |
1.5±0.8 |
−0.9±0.8 |
<0.001 |
|
Bothering group |
2.5±0.7 |
1.4±0.8 |
−1.1±0.8 |
<0.001 |
|
p-value (between groups) |
0.48 |
|
0.29 |
|
|
IPSS item 7 (nocturia) |
|
|
|
|
|
Non-bothering group |
2.4±0.9 |
1.7±0.9 |
−0.7±0.7 |
<0.001 |
|
Bothering group |
3.0±0.9 |
1.8±0.9 |
−1.1±0.9 |
<0.001 |
|
p-value |
<0.01 |
|
0.02 |
|
|
IPSS item 8 (quality of life) |
|
|
|
|
|
Non-bothering group |
3.4±1.2 |
2.5±1.3 |
−0.9±1.2 |
<0.001 |
|
Bothering group |
4.2±0.9 |
3.3±1.2 |
−1.0±1.2 |
<0.001 |
|
p-value |
<0.01 |
|
0.85 |
|
|
IPSS total scores |
|
|
|
|
|
Non-bothering group |
15.7±6.1 |
10.2±5.9 |
−5.8±5.1 |
<0.001 |
|
Bothering group |
18.3±6.1 |
13.1±6.2 |
−5.3±6.0 |
<0.001 |
|
p-value |
0.01 |
|
0.69 |
|
|
ICIQ N-2 (frequency of nocturia) |
|
|
|
|
|
Non-bothering group |
3.2±0.8 |
2.6±0.9 |
−0.7±0.9 |
<0.001 |
|
Bothering group |
3.7±0.9 |
2.7±0.8 |
−1.0±0.8 |
<0.001 |
|
p-value |
<0.01 |
|
0.05 |
|
|
ICIQ N-2b (bother due to nocturia) |
|
|
|
|
|
Non-bothering group |
3.9±2.7 |
2.7±2.3 |
−1.4±2.9 |
0.002 |
|
Bothering group |
6.9±2.4 |
4.6±2.6 |
−2.3±2.9 |
<0.001 |
|
p-value |
<0.01 |
|
0.12 |
|

Voiding symptom subscore was decreased by 3.0 in the non-bothering group and 3.0 in the bothering group. Difference in the decrease of voiding symptom subscores between the two groups was not statistically significant. Stroage symptom subscore was decreased by 2.2 in the non-bothering group and 2.3 in the bothering group. Difference in the decrease of storage subscore between the two groups was not statistically significant.
Quality of life score was decreased 0.9 in the non-bothering group and 1.0 in the bothering group. There was no significant difference in the decrease of quality of life score between the two groups.
2) Nocturia-related symptoms
Both groups showed significant reduction in discomfort due to nocturia. ICIQ-N 2b score was decreased from 3.9 to 2.7 (p=0.002) in the non-bothering group and from 6.9 to 4.6 in the bothering group (p<0.001) (
Fig. 2B). The degree of decrease in ICIQ-N 2b score was not significantly different between the two groups (p=0.12).
The number of nocturia episodes was significantly decreased in both groups. It was decreased from 2.4 to 1.5 (p<0.001) in the non-bothering group and from 2.5 to 1.4 in the bothering group (p<0.001). The difference in the frequency of nocturia reduction between the two groups was not significantly different (
Fig. 2C).
Go to :

DISCUSSION
Nocturia, one of the most bothersome LUTS, affects daily life activity and the quality of life [
125]. One of the consequences of nocturia is sleep deterioration, leading to increased daytime sleepiness and loss of energy and activity [
679]. Accidents including falls are increased both at night and during the day in the elderly who have nocturia [
8]. Therefore, when physicians treat patients with LUTS, they usually evaluate nocturia symptoms for appropriate management.
ICS defines nocturia as “the complaint that the individual has to wake at night one or more times to void… each void is preceded or followed by sleep” [
11]. This definition of nocturia is two-fold: One is the number of voidings at night and the other is complaint.
If a patient wakes at night one or more times to void without complaining about nocturnal voiding, physicians often ignore the symptoms without further evaluation or treatment. A few older patients also consider nocturia as part of natural aging [
12], without feeling bothered or seeking treatment. A prevalence study has shown that 36% of patients with two episodes of nocturia and 16% of patients with three episodes have no complaint [
13].
However, nocturia itself may lead to sleep disturbance, reduced daily activity, and even depression irrespective of patient's perceptions of nocturia [
14]. Treatment for nocturia may be required regardless of bothersomeness. However, there is no consensus or study determining the efficacy of treatment for non-bothering nocturia. This study showed that nocturia episodes were decreased in both groups irrespective of bothersomeness. Although the degree of decrease was significantly higher in the bothering group, the degree of discomfort measured by VAS was also significantly decreased in the non-bothering group. These results suggest that treatment reduced nocturia-related symptoms and discomfort regardless of subjective complaints.
One study about patients' perception on nocturia has shown that only 63% of patients visit physicians for nocturia [
12], probably because patients feel no discomfort as they may regard nocturia as a natural aging process. Therefore, they do not expect that nocturia can be reduced or cured. Patients often underestimate the damage of nocturia to sleep quality and daily activities. Results of this study suggest that information and education about nocturia should be provided to physicians and patients to identify their masked needs and improve nocturia.
This study has some limitations. First, this study was designed as a multicenter prospective study. However, treatment or drug therapy was not uniform among institutes. The optimal treatment was based on the decision of individual physician according to patients' characteristics. Due to the diverse etiology of nocturia in the study patients, no single medication was used to treat the patients. However, the aim of this study was not to evaluate the effect of a specified treatment but to demonstrate the value of treatment for patients who are not bothered by the symptoms or discomfort associated with nocturia. Second, we did not analyze the cause of nocturia. Nocturia can be caused by various conditions. Individual physicians evaluated the possible causes of nocturia and prescribed treatment according to patient characteristics. Third, the frequency of nocturia based on self-reported voiding diary might not be entirely accurate. Finally, the present study was a prospective study. However, the total number of study subjects was relatively small due to a high dropout rate. Nonetheless, baseline characteristics were comparable between the two groups and the number of study subjects was enough to demonstrate the decrease in nocturia discomfort in the non-bothering group.
Despite these limitations, this is the first prospective multicenter study showing that treatment for nocturia not perceived as an annoyance improved voiding symptoms and alleviated discomfort.
Go to :

CONCLUSIONS
Regardless of bothersomeness, both groups showed significant improvement in nocturia-related discomfort and voiding symptoms. Our results imply that treatment was beneficial for patients even if they were unaware of the discomfort caused by nocturia. Therefore, physicians and patients should not consider non-bothering nocturia as a natural process of aging or meaningless voiding symptoms. Instead, they should consider active treatment for nocturia.
Go to :

ACKNOWLEDGEMENTS
This study was sponsored by Astellas Pharma. The authors thank Kyungdo Han, Department of Medical Statistics, College of Medicine, The Catholic University of Korea, for his statistical assistance for this study.
Go to :

Notes
Go to :

References
1. Cornu JN, Abrams P, Chapple CR, Dmochowski RR, Lemack GE, Michel MC, et al. A contemporary assessment of nocturia: definition, epidemiology, pathophysiology, and management: a systematic review and meta-analysis. Eur Urol. 2012; 62:877–890. PMID:
22840350.
2. Van Kerrebroeck PE, Dmochowski R, FitzGerald MP, Hashim H, Norgaard JP, Robinson D, et al. Nocturia research: current status and future perspectives. Neurourol Urodyn. 2010; 29:623–628. PMID:
20432325.

3. Homma Y, Yamaguchi T, Kondo Y, Horie S, Takahashi S, Kitamura T. Significance of nocturia in the International Prostate Symptom Score for benign prostatic hyperplasia. J Urol. 2002; 167:172–176. PMID:
11743299.

4. Nimeh T, Alvarez P, Mufarreh N, Lerner LB. Nocturia: current evaluation and treatment for urology. Curr Urol Rep. 2015; 16:66. PMID:
26231263.

5. Park HK, Kim HG. Current evaluation and treatment of nocturia. Korean J Urol. 2013; 54:492–498. PMID:
23956822.

6. Barker JC, Mitteness LS. Nocturia in the elderly. Gerontologist. 1988; 28:99–104. PMID:
3342998.

7. Middelkoop HA, Smilde-van den, Neven AK, Kamphuisen HA, Springer CP. Subjective sleep characteristics of 1,485 males and females aged 50–93: effects of sex and age, and factors related to self-evaluated quality of sleep. J Gerontol A Biol Sci Med Sci. 1996; 51:M108–M115. PMID:
8630703.

8. Stewart RB, Moore MT, May FE, Marks RG, Hale WE. Noctu ria: a risk factor for falls in the elderly. J Am Geriatr Soc. 1992; 40:1217–1220. PMID:
1447437.
9. Klingler HC, Heidler H, Madersbacher H, Primus G. Nocturia: an Austrian study on the multifactorial etiology of this symptom. Neurourol Urodyn. 2009; 28:427–431. PMID:
19229953.

10. Robert G, Descazeaud A, Azzouzi R, Saussine C, Haillot O, Dumonceau O, et al. Impact of lower urinary tract symptoms on discomfort in men aged between 50 and 80 years. Urol Int. 2010; 84:424–429. PMID:
20339294.

11. van Kerrebroeck P, Abrams P, Chaikin D, Donovan J, Fonda D, Jackson S, et al. The standardisation of terminology in nocturia: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002; 21:179–183. PMID:
11857672.

12. Chen FY, Dai YT, Liu CK, Yu HJ, Liu CY, Chen TH. Perception of nocturia and medical consulting behavior among community-dwelling women. Int Urogynecol J Pelvic Floor Dysfunct. 2007; 18:431–436. PMID:
16874440.

13. Bing MH, Moller LA, Jennum P, Mortensen S, Skovgaard LT, Lose G. Prevalence and bother of nocturia, and causes of sleep interruption in a Danish population of men and women aged 60–80 years. BJU Int. 2006; 98:599–604. PMID:
16827903.

14. Obayashi K, Saeki K, Negoro H, Kurumatani N. Nocturia increases the incidence of depressive symptoms: a longitudinal study of the HEIJO-KYO cohort. BJU Int. 2017; 120:280–285. PMID:
28129482.
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download