INTRODUCTION
Varicocele is defined as abnormal dilatation of the pampiniform and/or cremasteric venous plexus. It is the most common treatable cause of male infertility, affecting roughly 15% to 20% of the general population and 40% of men presenting for an infertility evaluation. Varicocele is also the most common cause of secondary infertility, and has been reported in up to 80% of cases [
12]. In previous studies, varicocele was found to be associated with ipsilateral progressive testicular atrophy, deterioration of sperm parameters, decreased serum testosterone levels, and male infertility [
3].
The exact pathophysiologic mechanisms that cause varicocele-associated infertility have not been completely elucidated. The etiology and pathophysiology of varicocele can be multifactorial, including elevated testicular temperature, testicular hypoxia, oxidative stress (OS), and apoptosis [
45]. Studies have demonstrated that varicocele was associated with high levels of seminal OS, suggesting that impaired sperm function could be related to OS in patients' semen [
67].
Reactive oxygen species (ROS) include the superoxide anion (O
2•
−), hydrogen peroxide (H
2O
2), and the hydroxyl radical (•OH). ROS are products of normal cellular metabolism and produced by both seminal leukocytes and abnormal sperm in semen. Low levels of ROS are necessary for sperm capacitation, the acrosome reaction, hyperactivation, and fusion with the oocyte [
8910]. However, in certain pathological cellular conditions, supra-physiologic ROS production overwhelms the antioxidant defense mechanisms, resulting in OS. Excessive ROS increase the peroxidation of the polyunsaturated fatty acids of the sperm cell membrane and can damage spermatozoa. ROS can also induce sperm DNA fragmentation and apoptosis in mature spermatozoa [
1112]. These findings may explain the clinical presentation of impaired semen parameters in men with varicocele and elevated OS.
To date, most clinical studies have investigated total ROS production in the semen of patients with varicocele. To the best of our knowledge, only 1 clinical study in the literature has investigated superoxide anion production in the untreated semen of patients with varicocele [
13]. This prompted us to assay superoxide anion production by the spermatozoa of patients with varicocele using the lucigenin-dependent chemiluminescence (CL) method.
The objective of this study was to investigate the pathophysiological role of superoxide anion and total ROS production by the spermatozoa of men with varicocele and its relationship with varicocele grade and semen parameters.
Go to :

MATERIALS AND METHODS
1. Ethics statement
The present study protocol was reviewed and approved by the Institutional Review Board of Okmeydanı Training and Research Hospital (Reg. No. 688). Informed consent was submitted by all subjects when they were enrolled.
2. Study design and patient selection criteria
This multicenter prospective study was conducted between January and December 2017. Patients who were admitted to Okmeydanı Hospital outpatient clinic with the complaint of left scrotal discomfort were evaluated with a detailed history, physical examination, scrotal Doppler ultrasound, and measurement of serum follicle-stimulating hormone (1.27–19.26 mIU/mL) and total testosterone (1.75–7.81 ng/mL) levels. Varicoceles were clinically graded as grade II (palpable while standing upright) or grade III (visible through the scrotal skin) [
314]. The inclusion criteria for the study were as follows: men aged between 18 and 35 years with clinical grade II/III varicocele on physical examination, as also confirmed by Doppler ultrasound (with the finding of 1 or more veins with a maximal diameter >3 mm and retrograde flow seen either at rest or during the Valsalva maneuver) and normal serum hormone levels, who were non-smokers, had no history of varicocele surgery, genitourinary infections, or medical treatment with antioxidants in last 6 months. All participants were evaluated using semen analysis and ROS assays. Patients with azoospermia and leukocytospermia on semen analysis were excluded from the study. A total of 34 men who met the inclusion criteria comprised the patient group. The control group included 13 age-matched healthy men with a normal semen analysis who had no history of smoking, urinary infection/prostatitis, or varicocele surgery, and no varicocele on physical examination.
3. Semen analysis
Semen samples were collected from participants through masturbation into sterile containers after 4 to 5 days of sexual abstinence. After liquefaction, semen specimens were evaluated for semen volume, appearance, and viscosity. Semen characteristics were examined according to the 2010 World Health Organization criteria [
15].
4. White blood cells in semen
The presence of leukocytes in semen specimens was assessed by a Leucoscreen (Fertipro, Beernem, Belgium) test. Leukocytospermia was defined as the presence of at least 1.0×106 white blood cells (WBCs) per milliliter.
5. Swim-up method
After liquefaction, the sample was transferred into 4 sterile 15-mL tubes using a plastic pipette, and the weight was measured. The specimen was mixed with the same amount of human tubal fluid (HTF; Lifeglobal, Guelph, ON, Canada) +5% protein medium using a Pasteur pipette. The suspended sample was transferred into 4 sterile 15-mL tubes using plastic pipettes and centrifuged at 800 rpm for 10 minutes. The supernatant was aspirated and discharged. The samples were mixed with 0.5 mL of fresh HTF medium for each tube using a Pasteur pipette and centrifuged at 800 rpm for 10 minutes. The supernatant was aspirated and discharged, after which 0.25 mL of HTF medium was slowly added without compromising the pellet in each tube. The tubes were then incubated at an angle of 45 for 1 hour in the incubator at 37℃. After the incubation period, the supernatant was transferred to a sterile 5-mL tube using a Pasteur pipette.
6. Determination of reactive oxygen species
Total ROS and superoxide anion levels were measured by the CL method using a luminometer (LB9509; Berthold, Bad Wildbad, Germany) at room temperature. The sperm pellets were re-suspended in the same medium at a concentration of 1.0×106 spermatozoa/mL. Luminol (5-amino-2,3-dihydro-1,4-phthalazinedione) or lucigenin (10,10′-dimethyl-9,9′-biacridinium dinitrate) enhancers were added to the test tubes for a final concentration of 0.2 mM. Superoxide anion production was measured by lucigenin-dependent CL. In contrast, luminol was used to detect a broader range of reactive species, (i.e., •OH, H2O2, and HOCl radicals). Counts were made for 5 minutes at 1-minute intervals. After the measurements, the area under the curve was calculated and corrected for the sperm count. Results were given as relative light unit (RLU)/1.0×106 spermatozoa/mL. In order to normalize values to the same distribution, log transformed values (ROS+1) of the results were used in calculations.
7. Statistical analysis
The statistical results are presented as mean±standard deviation. The distribution of variables was assessed with the Kolmogorov-Smirnov test. The Mann-Whitney U-test was used to compare independent quantitative data. The Kruskal-Wallis test was used for multiple comparisons. Correlations between variables were calculated using the Spearman method. The power of the study was calculated using the G*Power program (University of Dusseldorf, Dusseldorf, Germany) with an effect size convention of 0.8 for the 2-tailed t-test, with an alpha error protection of 0.05. Statistical significance was assessed using 2-tailed tests, and p-values <0.05 were considered to indicate statistical significance. Statistical tests were performed using IBM SPSS ver. 22.0 (IBM Co., Armonk, NY, USA).
Go to :

DISCUSSION
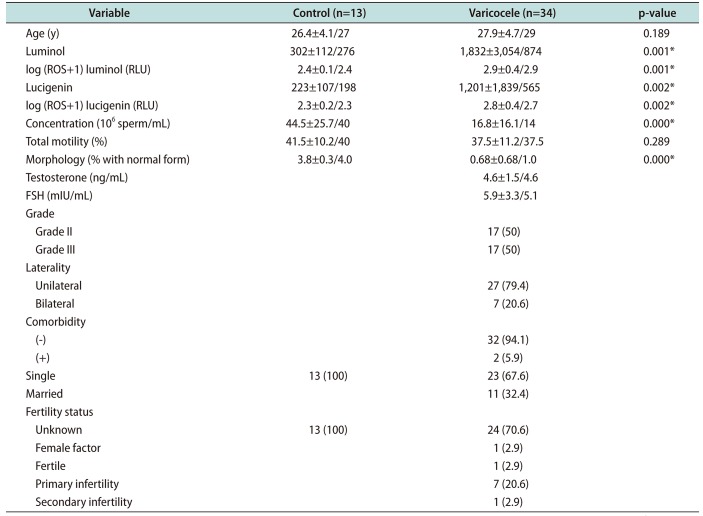
In the present study, we found that lucigenin-dependent superoxide anion levels were higher in patients with varicocele than in control subjects. To the best of our knowledge, this is the first study to demonstrate increased superoxide anion production by the spermatozoa of patients with varicocele assayed using the lucigenin-dependent CL method. A CL assay utilizing luminol is the most commonly described direct method to measure ROS production by the spermatozoa. The amount of oxidation in the spermatozoa cell membrane shows the net oxidative imbalance between ROS production and the antioxidant levels in semen [
16]. The luminol-dependent CL assay measures intracellular and extracellular ROS levels (O2•
−, •OH, H
2O
2) because luminol reacts with a variety of ROS. However, this method does not discriminate these oxidants from one another, meaning that it measures total ROS levels. Polymorphonuclear leukocyte ROS production accounts for most of the total ROS production in CL assays in untreated semen. Various semen-washing methods can be used to eliminate WBCs from semen in order to assay spermatozoal ROS production. H
2O
2 is more reactive than superoxide anion in the luminol-dependent CL method. Lucigenin-dependent CL is a more specific and validated method for assaying extracellular superoxide anion levels [
17].
Many studies have measured seminal ROS levels in infertile men with varicocele and compared the values with normospermic or fertile donors [
67]. Those studies, which used a luminol-dependent CL method, showed that ROS levels were significantly higher in varicocele patients than in healthy controls. Our results, showing that patients with varicocele had higher total ROS levels than controls, are compatible with the literature. In fact, most of the studies in the literature about OS in male infertility and varicocele have used luminol-dependent CL or lipid peroxidation products (
e.g., malondialdehyde; MDA) and oxidized DNA assays, which are indirect methods of measuring ROS/OS [
671819]. Indirect methods measure the end products of the peroxidative process or DNA damage, and mostly provide information about ROS-related damage. Considering those facts, we preferred to use the CL method to assay ROS production by the spermatozoa of patients with varicocele. For the first time, we used a lucigenin enhancer to assay spermatozoal superoxide anion production in washed semen samples from varicocele patients, unlike other studies that only utilized a luminol enhancer.
Mazzilli et al [
13] studied O
2•
− overproduction in semen from 152 subjects, including 18 patients with varicocele. They reported that 88.9% of the varicocele patients had increased semen O
2•
− production. Superoxide anion levels were significantly higher in patients with varicocele than in normospermic subjects in their study. They also reported that a close correlation was observed between O
2•
− levels and WBCs, non-rapid immotile sperm, and sperm abnormalities. That was the only study in the literature that showed O
2•
− overproduction in the semen of patients with varicocele. They used untreated semen to assay O
2•
−, meaning that they measured total O
2•
− production from spermatozoa and infiltrating WBCs in the semen. Since antioxidant enzymes are present in seminal plasma, it can be supposed that increased O
2•
− production overwhelmed the protective antioxidant properties of the seminal plasma of patients with varicocele in their study. Instead, in our study, we washed semen samples using the swim-up procedure to eliminate WBCs, which make a major contribution to ROS production from semen. Therefore, our aim was to assay spermatozoal O
2•
− production by reducing the number of WBCs in the semen samples of participants. The other difference was the methodology used for assaying O
2•
−. Mazzilli et al [
13] used cytochrome c reduction in their methodology, whereas we used lucigenin-dependent CL.
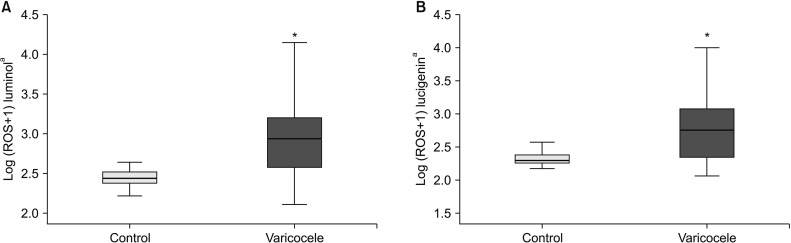
Several studies have demonstrated varicocele grade to be correlated with seminal ROS levels. Mostafa et al [
18] showed that seminal MDA and H
2O
2 levels were significantly higher in patients with grade II or III varicocele than in grade I patients. Allamaneni et al [
20] demonstrated that luminol-dependent ROS levels were significantly higher in patients with grade II or III varicocele than in men with grade I varicocele. We found that total ROS and superoxide levels of patients with grade III varicocele were significantly higher than the grade II patients and control subjects. Sperm concentrations and the proportion of normal sperm morphology were significantly lower in patients with grade III varicocele than in patients with grade II varicocele in our study. Our results suggest that varicocele grade III is associated with higher ROS levels and has a more detrimental effect on sperm concentration and morphology than grade II varicocele.
Animal studies in varicocele models could provide leads for understanding the cellular pathophysiological mechanisms of varicocele in humans. Cam et al [
21] reported that testicular tissue O2•
− levels were higher in a rat left varicocele model group than in the corresponding control group. Lucigenin-dependent CL levels were significantly higher in the left testicles of the varicocele group than in the sham group in their study. They also found a higher apoptotic index in testicular tissue in an experimental varicocele model. Jafari et al [
22] showed that mitochondrial superoxide anion production assayed by the flow cytometric method was the main source of ROS production by sperm cells in a rat left varicocele model. They found higher sperm intracellular O2•
− production and lower mitochondrial membrane potential, sperm count, viability, and motility in rats with experimental left varicocele. They suggested that increased intracellular O2•
− production could be an important mechanism in mitochondrial dysfunction in spermatozoa, resulting in functional abnormalities and the death of sperm cells in a varicocele rat model. Our results are compatible with those experimental studies, as we found that spermatozoal O2•
− anion production levels were higher in patients with varicocele than in control subjects and that O2•
− anion levels were negatively correlated with the semen parameters of patients with varicocele.
The main source of ROS production in somatic cells is electron leakage from the mitochondrial electron transport chain (ETC) during cellular respiration. Superoxide anion is the primary ROS generated during cellular respiration, and it is produced through a monovalent reduction of oxygen via the addition of a single electron. Aitken et al [
23] reported that the primary ROS produced by human spermatozoa was superoxide anion, which is converted into hydrogen peroxide in a reaction catalyzed by superoxide dismutase. Superoxide can be generated at complex I, II, or III in the ETC in the mitochondria [
24]. Studies have suggested that hypoxia stimulates ROS production by complex I and III in the mitochondrial ETC [
2526]. Agarwal et al [
27] emphasized the importance of hypoxia and hyperthermia in the pathophysiology of varicocele. They proposed that varicocele is essentially associated with a state of energy deprivation, hypoxia, and hyperthermia due to decreased blood supply. They suggested that hypoxiainduced ROS release could be responsible factor for the varicocele pathophysiology because the hypoxia sensor complex III of the ETC was downregulated in their study. Since superoxide generation by the ETC increases during hypoxia, the increased O2•
− levels in our patient group support their hypothesis regarding the importance of sperm mitochondrial dysfunction in the pathophysiology of varicocele.
The studies discussed above, in conjunction with our study, suggest that hypoxia-induced mitochondrial dysfunction and increased mitochondrial O2•−/ROS production by the spermatozoa may be responsible for defective sperm function in patients with varicocele.
There are several limitations of our study. First, the study was conducted with a small sample size, which is important drawback despite its prospective design. Unlike other studies, we did not find a statistically significant difference in sperm motility between the varicocele patients and the control group. Our different findings for the motility parameter could have been because the mean percentage of total sperm motility was at the lower reference limits in the control group, and there was a limited number of individuals in the study cohort. Sperm motility is known to be a major parameter that is negatively affected by ROS overproduction, as has been demonstrated in many studies. In fact, there was a negative correlation between total ROS/superoxide anion levels and motility in our study group, which could be a significant indicator of the harmful effect of ROS on sperm motility. Second, we did not investigate the ameliorative effect of antioxidant supplementation on O2•− anion/ROS overproduction or the varicocele-induced detrimental impact on semen parameters in our patient group. Such research, in the form of clinical studies, would help clinicians gain an understanding of the underlying pathophysiologic mechanisms of varicocele. The main clinical implication of our study is the possible therapeutic role of antioxidant treatment (monotherapy or adjuvant to varicocelectomy) for the treatment of impairment of semen parameters and resulting infertility in varicocele patients. Therefore, future studies are needed to clarify the efficacy of antioxidant supplementation to ameliorate ROS-related damage to the spermatozoa in the varicocele patients.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download