Abstract
Purpose
This study was performed to evaluate and compare threshold sperm parameters and sperm DNA fragmentation index (DFI), and further analyzed whether sperm DFI could be predicted from sperm parameters in men with varicocele.
Materials and Methods
A total of 157 semen samples underwent both semen analysis and sperm DNA fragmentation (SDF) testing in men with varicocele. Sperm parameters were assessed using the World Health Organization guidelines. SDF testing was performed using the Halosperm kit. Sperm parameters and sperm DFI results were compared.
Results
The overall sperm parameter results and sperm DFI showed normal values; however, the morphology value was at the lower limit of normal. High sperm DFI was associated with significantly lower motility and viability (p<0.001, respectively). Sperm motility and morphology were significantly higher in the higher sperm count group compared to the lower sperm count group (p<0.05), while sperm DFI was higher in the lower sperm count group (p<0.05). Sperm count and viability and sperm DFI were significantly associated with the quality of sperm motility (p<0.001). Sperm motility and sperm DFI were significantly different (p<0.001) between normal and abnormal sperm viability groups. Between normal and abnormal sperm morphology groups, sperm count, motility, and sperm DFI showed significant differences (p<0.001).
Conclusions
In this study, a correlation between SDF and sperm parameters was confirmed in men with varicocele. SDF may be contributing factors to sperm motility, viability, and morphology. Abnormal sperm count, motility, and viability showed high sperm DFI. Therefore, lower sperm parameters were indicative of increasing SDF in men with varicocele.
Traditionally male infertility has been diagnosed based on semen analysis, including semen volume, pH, morphology, motility, and concentration. The one of the most main cause of male factor infertility is varicocele [1]. The term varicocele has been used to describe pathological abnormal dilations of the testicular veins in the pampiniform plexus and by retrograde blood flow in the valves [2]. Patients with varicocele present with abnormal spermatogenesis, but the pathophysiological mechanism by which varicocele can induce male factor infertility remains unclear, although several potential causes have been postulated [3]. In a World Health Organization report [4], a varicocele was identified in 25.4% of men with abnormal sperm parameters compared with only 11.7% of men with normal sperm parameters.
Varicocele can be treated either by microscopic surgical varicocelectomy, which is considered the gold-standard approach to varicocele repair [5]. However, there are many conflicting reports. In most studies, sperm concentration improved after varicocelectomy [67], but other studies showed no improvement following surgery [89]. Clearly, there are differing opinions whether or not varicocelectomy improves fertility. Furthermore, subtle forms of spermatozoa dysfunction with lower biological variability, such as DNA integrity, can be used to assess the effectiveness of varicocelectomy [10].
Recently, sperm DNA integrity has been recognized as a new potential fertility predictor and a potential parameter of sperm quality. Several studies reported the relationship between sperm DNA damage and varicocele [1112], while others showed that sperm DNA damage is associated with impaired fertilization, embryo cleavage and embryo quality, miscarriage, and recurrent pregnancy loss after in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) [13]. Sperm quality could be associated with sperm DNA fragmentation (SDF) in infertile patients. Sperm DNA damage appears at high frequencies in abnormal semen and sperm DNA integrity is an important indicator of normal sperm.
Over the past two decades, sperm morphology has been recognized as an important predictor of outcomes in artificial intrauterine insemination, conventional IVF, and ICSI [14]. While the relationship between sperm concentration and SDF in subfertile men appears to vary, a correlation between SDF and sperm viability has been demonstrated [15]. Although the mechanisms have not been fully recognized, SDF is seen in mature, viable sperm. DNA integrity appears to be a biomarker of sperm quality, and sperm DNA damage could thus be a potential predictor for male infertility. However, sperm DNA integrity is not assessed as a routine part of semen analysis in clinical laboratories.
The purpose of this study was to evaluate and compare threshold sperm parameters and sperm DNA fragmentation index (DFI) in men with varicocele. To focus on the effects of sperm parameters affecting DFI value, sperm parameters were divided as count, motility, morphology, viability and further analyzed whether the normality of sperm DFI could be predicted using these individual parameters.
A total of 157 semen samples were included in semen analysis and SDF testing during January 2015 to March 2017. All included patients visited to Department of Urology, Cheil General Hospital & Women's Healthcare Center, who had no baby at least 1 year after try to conceive. We excluded patients with other disease affect to male fertility and normal semen parameters, only include patients with varicocele and abnormal semen parameters. Each patient underwent physical examination and hormonal profile testing. In physical examination, all enrolled patients were confirmed varicocele and also rechecked with scrotal ultrasound test.
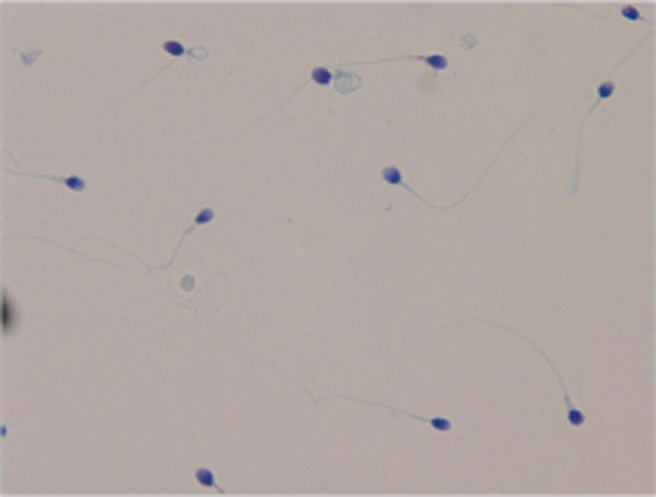
Semen was collected by masturbation into a sterile plastic cup after 3 to 5 days of sexual abstinence. The semen specimen was left for at least 30 minutes at room temperature for liquefaction. Semen analysis was based on World Health Organization [16] criteria. Sperm count and motility was objectively assessed using a Makler counting chamber with a computerassisted sperm analyzer (Sperm Analysis Imaging System [SAIS], Seoul, Korea). The viability was evaluated using eosin-nigrosin staining. Sperm were classified as viable if the sperm head was unstained and as non-viable if the sperm head stained red-pink (Fig. 1). Evaluation of sperm morphology was performed using the Papanicolaou staining method (Fig. 2). The Papanicolaou staining enables classification of head defects with vacuoles of spermatozoa and other cells; sperm stains pale blue in the acrosomal region and dark blue in the post-acrosomal regions of the head. Excess residual cytoplasm stains pink or red. The midpiece may show some red staining and the tail is stained blue or reddish. The acrosomal region should contain no large vacuoles, and not more than two small vacuoles, which should not occupy more than 20% of the sperm head. The post-acrosomal region should not contain any vacuoles. The lower reference limit for sperm parameters is as follows: sperm count ≥15×106 sperm/mL, motility ≥40%, viability ≥58%, normal morphology ≥4%. Sperm parameters were divided into two classes: the upper reference limits consider to normal sperm parameter and lower reference limits consider to abnormal sperm parameter. Also, for the evaluate sperm DFI with sperm parameters, sperm parameter groups were divided into two groups according to the 30% of sperm DFI. If sperm count was lower than <5×106 sperm/mL, sperm morphology and viability were not analyzed.
Sperm DNA fragmentation was assessed using the Halosperm kit (Halotech DNA, S.L., Madrid, Spain) according to the manufacturer's instructions. Briefly, semen sample was diluted to 5–10×106/mL in sperm washing medium (SAGE; CooperSurgical, Inc., Trumbull, CT, USA). An agarose-containing Eppendorf tube was floated in water for 5 minutes at 90℃ to 100℃, until the agarose dissolved. The agarose tube was transferred to a temperature-controlled water bath maintained at 37℃ and left for 5 minutes until the temperature was even. Then, 25 µL of the diluted semen sample was transferred to the melted agarose tube and mixed well. Immediately, 10 µL of the mixed cell suspension was placed on a coated slide glass (provided by the kit) and covered with a coverslip. The slide was then placed on a cold plate for 5 minutes at 4℃ in order for the agarose to solidify with the spermatozoa embedded within. The coverslip was then gently removed and the slide was fully immersed in denaturant solution, and placed horizontal to incubate for 7 minutes at room temperature. To make the denaturant solution, 80 µL of the acid denaturant solution (provided by the kit) was added to 10 mL of distilled water and mixed well. Afterwards, the slide was horizontally immersed in a lysis solution and incubated for 25 minutes. The slide was moved into abundant distilled water for 5 minutes in order to wash the lysis solution. The slide was placed horizontal in a tray and rinsed in an ethanol series (70%, 90%, and 100%) for 2 minutes in a stepwise manner and then air-dried. For visualization, Diff-Quik staining was used. The slide was immersed in eosin solution (red color) for 7 minutes. The slide was then move into Azur B solution (blue color) for 7 minutes, washed and allowed to dry. The slide was covered with a coverslip and spermatozoa were observed with a halo under a bright microscope (×200). For spermatozoa classification of DNA fragmentation, spermatozoa with a large- or medium-sized halo were considered without fragmentation (normal spermatozoa) and spermatozoa with a small halo, without a halo or that were degraded were considered to exhibit fragmentation (DNA-fragmented spermatozoa) (Fig. 3). All SDF analysis was performed and analyzed by same expert senior andrologist. A total of 500 spermatozoa per sample were scored. The fragmentation rate were calculated as the DFI (%)=(fragmented spermatozoa/total spermatozoa counted)×100. A threshold sperm DFI value exceeding 30% was considered abnormal according to the manufacturer's instructions.
This study was approved by the Institutional Review Board of Cheil General Hospital and Women's Healthcare Center, Seoul, Korea (approved No. CGH-IRB-2017-30).
Data are shown as mean±standard deviation. Numerical data for the two groups of interest were compared by investigating their degree of correlation and performing the Student's t-test. Correlation coefficient among SDF and semen parameters was calculated via Pearson methods. Differences were considered statistically significant at p<0.05.
Regarding the overall results for sperm parameters, normal and abnormal sperm DFI was evaluated (Table 1). For the evaluate sperm parameter with sperm DFI, sperm parameters were categorized according to a threshold sperm DFI value of 30%. The total results of sperm parameters and sperm DFI were within normal range; however, morphology values were at the lower limit of normal and showed broad range (range, 0–22). For the normal sperm DFI group (≤30%) and the abnormal sperm DFI group (>30%), mean age was 34.4±4.0 years and 36.0±5.3 years, respectively (p<0.05). Mean sperm DFI was 18.8%±6.2% in the normal sperm DFI group, and 45.1%±17.6% in abnormal sperm DFI group. In abnormal sperm DFI group, the average sperm count (range, 1–284.3×106 sperm/mL), and average sperm morphology (0%–15%) showed broad value, and statistical difference was not observed. High sperm DFI was associated with significantly lower motility and viability (p<0.001). Sperm parameters showed normal value in normal sperm DFI group. Although sperm morphology showed lower value in abnormal sperm DFI group, statistical difference was not observed. A comparison of sperm parameters and sperm DFI between the normal (≥15×106 sperm/mL) and abnormal (<15×106 sperm/mL) sperm count groups is shown in Table 2. Sperm motility and morphology were significantly better in the normal sperm count group (p<0.001 and p<0.05, respectively), while sperm DFI was higher in the abnormal sperm count group (24.5%±13.1% vs. 33.5%±24.9%, p<0.05). In this result, if sperm count was lower than <5×106 sperm/mL, sperm morphology and viability were not analyzed. Therefore, broad range of standard deviation was showed in abnormal morphology group. Although viability was not significantly different, sperm count affect to sperm parameters and sperm DFI.
Table 3 shows the relationship of normal sperm motility (≥40%) and abnormal sperm motility (<40%) with sperm parameters and sperm DFI. Sperm count and viability were significantly associated with sperm motility (p<0.001). Sperm DFI also varied significantly between normal and abnormal sperm motility groups (20.0%±8.8% vs. 30.6±18.4%, p<0.001). In abnormal sperm DFI group, the average sperm count (range, 1–330.5×106 sperm/mL), and average sperm morphology (0%–16%) showed broad value. Sperm parameters and sperm DFI with respect to normal sperm viability (≥58%) and abnormal sperm viability (<58%) are presented in Table 4. Sperm viability was not associated with sperm count or morphology. Sperm motility and sperm DFI were significantly different (p<0.001) between normal and abnormal sperm viability groups. Sperm morphology value in abnormal sperm DFI group showed broad range (0%–17%).
In Table 5, sperm count and motility showed statistically significant differences between the normal sperm morphology (≥4%) and abnormal sperm morphology (<4%) groups (p<0.001), while sperm viability and DFI were not shown significantly different within normal range. This result supposed to sperm DFI does not affected by sperm morphology. In the overall results for sperm parameters, Pearson's analysis observed that correlation coefficient among sperm parameters, motility and viability presented a negative and significant correlation with sperm DFI (Table 6).
The frequency of SDF has been recognized as a new potential parameter of semen quality. Increased SDF rates are correlated with severe sperm defects, male infertility, poor fertilization rates, poor embryonic development, decreased implantation rates, low pregnancy rates, and increased risk of pregnancy loss after assisted reproductive technology (ART) [1718]. The etiology of sperm DNA damage seems to be multifactorial and may result from intrinsic and/or external factors. In the present study, motility, viability, and morphology were significantly lower in the >30% sperm DFI group (p<0.05). As a rule, increased SDF is correlated with poor sperm morphology; in addition, sperm with normal morphology and motility tend to exhibit a low DNA damage rate. This suggests that performing ICSI using sperm with high DNA fragmentation rates would lead to similar fertilization and clinical pregnancy rates as ICSI with sperm with low DNA fragmentation rates [19].
Several hypotheses have been proposed to explain the origin of sperm DNA damage. In a meta-analysis, an increase in sperm DNA damage was revealed in men with varicoceles [20]. Data in the literature have demonstrated an increase in DNA fragmentation in men with varicocele and a clear association between oxidative stress and worse sperm parameters, including conventional parameters and sperm DNA damage [21]. Dieamant et al [3] demonstrated that men with varicocele had lower total sperm count, inferior progressive and total sperm motility, reduced vitality, and abnormal morphology compared to a control group. This study demonstrated that SDF was increased in varicocele group compared to the control group. In men with varicocele, impaired spermatogenesis, decreased progressive motility, and reduced sperm concentration are the most common abnormalities [22] and may cause deleterious alterations in early spermatid head differentiation during spermiogenesis, suggesting that varicocele patients with a high incidence of sperm acrosome and nucleus malformation are good candidates for varicocelectomy [2022]. Enciso et al [23] reported that men with varicocele exhibit a higher yield of sperm cells with the greatest nuclear DNA damage in a population with fragmented DNA. García-Peiró et al [12] reported a significant increase in DNA stainability and in DNA-degraded spermatozoa in the presence of varicocele. Another potential explanation for DNA damage in men with varicocele might be the elevated intratesticular temperature that is associated with varicocele, which can affect testicular function [24].
The sperm chromatin integrity test has been suggested to be useful in the assessment of varicocele [9]. Although studies have reported different clinical values, several specific tests have been developed to determine SDF: the sperm chromatin structure assay (SCSA) using flow cytometry, TdT-mediated dUTP nick end labeling (TUNEL) assay, and the single-cell gel electrophoresis COMET assay. However, these procedures cannot be performed routinely in conventional semen analysis laboratories because they are complex, difficult to perform, time-consuming and relatively expensive. SCSA is a statistically robust test, but not all laboratories have access to a flow cytometer or the technical expertise needed to perform this assay. Therefore, any technique used to analyze SDF in a clinical andrology or ART laboratory should be simple and reproducible. With regard to sperm DFI, in several studies a DNA fragmentation rate of ≥30% was associated with impaired fertility outcomes and increased spontaneous abortion rates [25]. Another report showed that a high percentage of damaged spermatozoa with denatured DNA (>30%) led to very low fertility potential [26]. In our study, a threshold sperm DFI value exceeding 30% was considered abnormal according to the manufacturer's instruction. All SDF analysis was performed and analyzed by same expert senior andrologist. Therefore, inter-observer variation was not present.
A new procedure for the determination of SDF, the sperm chromatin dispersion (SCD) test, has been developed. The SCD test enables detection of different degrees of nuclear DNA damage and discrimination of sperm nuclei with fragmented DNA [27]. In the SCD protocol, sperm nucleoids can be visualized using a fluorescence microscope or bright microscopy after Diff-Quik staining. In the initial protocol, although halos can be seen using Diff-Quik staining and bright microscopy, the staining results are very weak and with poor contrast. Stained objects show low nucleoid density more faintly and with less contrast. If the chromatin is less dense, the peripheral limit of the halo may not be accurately discriminated from the background. This phenomenon can cause errors when quantifying halo size. If sperm tails are not present in the stained sample, discrimination from other cell types can be difficult. Thus, there are limitations associated with bright field microscopy of Diff-Quik staining. However, the initial SCD protocol has been improved, and assessment of sperm cell nuclear halo size and distinction from non-germ cell types can be accurately and confidently performed in every basic laboratory using Diff-Quik staining and conventional bright field microscopy [28]. The modified SCD test, known as the Halosperm kit, assesses the capacity of sperm chromatin to disperse under hydrochloric acid denaturation of the chromatin that detected only single-strand DNA. After denaturing, a lysing solution was used to remove excess nuclear proteins. The level of fragmentation can be estimated based on the size of the nuclear dispersion and measured using optic microscopy. The SCD test gives predictive values for SDF similar to the SCSA and TUNEL and is relatively easy, expeditious, and reproducible. Therefore, in our study, we used the modified SCD test (Halosperm) kit. Using a threshold sperm DFI value of 30%, significant differences in motility, viability, and morphology were observed (p<0.001, Table 1). Semen with values near the lower limit of normal for count, motility, viability, and morphology had significantly higher sperm DFI. Sellami et al [29] found no correlation between the degree of sperm chromatin condensation and sperm parameters including sperm count, motility, and viability. In our study, we can suggest that chromatin condensation constitutes a useful parameter in the assessment of male fertility, completely independent of conventional sperm parameters, however, we focused on the relationship between SDF value and sperm parameters, an optimal predictive indicator for the ART outcomes was not identified. Also, a potential weakness of the present study is that a limited number of semen analysis samples were used to produce these results, which may have confounded the results. Therefore, a larger number of semen samples will be needed in future studies.
In conclusion, SDF may be contributing factors to sperm motility, viability, and morphology. Also, abnormal sperm count, motility, and viability showed high sperm DFI value. The relationship between SDF and abnormal sperm parameters was confirmed in men with varicocele. Therefore, abnormal sperm parameters were indicative of increasing SDF in men with varicocele.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant No. HI14C0106).
Notes
This paper was presented at the 73rd Annual Meeting of the Korean Society for Reproductive Medicine, November 25, 2017, Seongnam, Republic of Korea.
Author Contribution: Research conception & design: Park YS, Seo JT. Performing the experiments: Park YS, Choi HW, Lee SH. Data acquisition: Lee HS, Lee JS, Seo JT. Data analysis and interpretation: Park YS, Lee HS, Seo JT. Drafting of the manuscript: Park YS, Seo JT. Critical revision of the manuscript: Park YS, Seo JT. Approval of final manuscript: all authors.
References
1. Kohn TP, Kohn JR, Pastuszak AW. Varicocelectomy before assisted reproductive technology: are outcomes improved? Fertil Steril. 2017; 108:385–391. PMID: 28865536.

2. Gat Y, Bachar GN, Zukerman Z, Belenky A, Gornish M. Varicocele: a bilateral disease. Fertil Steril. 2004; 81:424–429. PMID: 14967384.

3. Dieamant F, Petersen CG, Mauri AL, Conmar V, Mattila M, Vagnini LD, et al. Semen parameters in men with varicocele: DNA fragmentation, chromatin packaging, mitochondrial membrane potential, and apoptosis. JBRA Assist Reprod. 2017; 21:295–301. PMID: 29068181.

4. World Health Organization. The influence of varicocele on parameters of fertility in a large group of men presenting to infertility clinics. Fertil Steril. 1992; 57:1289–1293. PMID: 1601152.
5. Grober ED, O'brien J, Jarvi KA, Zini A. Preservation of testicular arteries during subinguinal microsurgical varicocelectomy: clinical considerations. J Androl. 2004; 25:740–743. PMID: 15292104.

6. Madgar I, Weissenberg R, Lunenfeld B, Karasik A, Goldwasser B. Controlled trial of high spermatic vein ligation for varicocele in infertile men. Fertil Steril. 1995; 63:120–124. PMID: 7805900.
7. Onozawa M, Endo F, Suetomi T, Takeshima H, Akaza H. Clinical study of varicocele: statistical analysis and the results of long-term follow-up. Int J Urol. 2002; 9:455–461. PMID: 12225343.

8. Kamischke A, Nieschlag E. Varicocele treatment in the light of evidence-based andrology. Hum Reprod Update. 2001; 7:65–69. PMID: 11212077.

9. Zini A, Blumenfeld A, Libman J, Willis J. Beneficial effect of microsurgical varicocelectomy on human sperm DNA integrity. Hum Reprod. 2005; 20:1018–1021. PMID: 15608026.

10. Zini A, Kamal K, Phang D, Willis J, Jarvi K. Biologic variability of sperm DNA denaturation in infertile men. Urology. 2001; 58:258–261. PMID: 11489713.

11. Wu GJ, Chang FW, Lee SS, Cheng YY, Chen CH, Chen IC. Apoptosis-related phenotype of ejaculated spermatozoa in patients with varicocele. Fertil Steril. 2009; 91:831–837. PMID: 18314115.

12. García-Peiró A, Oliver-Bonet M, Navarro J, Abad C, Amengual MJ, López-Fernández C, et al. Differential clustering of sperm subpopulations in infertile males with clinical varicocele and carriers of rearranged genomes. J Androl. 2012; 33:361–367. PMID: 21835912.
13. Esbert M, Pacheco A, Vidal F, Florensa M, Riqueros M, Ballesteros A, et al. Impact of sperm DNA fragmentation on the outcome of IVF with own or donated oocytes. Reprod Biomed Online. 2011; 23:704–710. PMID: 22019617.

14. Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 1988; 49:112–117. PMID: 3335257.

15. Brahem S, Jellad S, Ibala S, Saad A, Mehdi M. DNA fragmentation status in patients with necrozoospermia. Syst Biol Reprod Med. 2012; 58:319–323. PMID: 22871031.

16. World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: WHO;2010.
17. Chohan KR, Griffin JT, Lafromboise M, De Jonge CJ, Carrell DT. Comparison of chromatin assays for DNA fragmentation evaluation in human sperm. J Androl. 2006; 27:53–59. PMID: 16400078.

18. Henkel R, Hajimohammad M, Stalf T, Hoogendijk C, Mehnert C, Menkveld R, et al. Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertil Steril. 2004; 81:965–972. PMID: 15066449.

19. Dar S, Grover SA, Moskovtsev SI, Swanson S, Baratz A, Librach CL. In vitro fertilization-intracytoplasmic sperm injection outcome in patients with a markedly high DNA fragmentation index (>50%). Fertil Steril. 2013; 100:75–80. PMID: 23562046.
20. Wang YJ, Zhang RQ, Lin YJ, Zhang RG, Zhang WL. Relationship between varicocele and sperm DNA damage and the effect of varicocele repair: a meta-analysis. Reprod Biomed Online. 2012; 25:307–314. PMID: 22809864.

21. Nasr Esfahani MH, Tavalaee M. Origin and role of DNA damage in varicocele. Int J Fertil Steril. 2012; 6:141–146. PMID: 24520431.
22. Nasr-Esfahani MH, Abasi H, Razavi S, Ashrafi S, Tavalaee M. Varicocelectomy: semen parameters and protamine deficiency. Int J Androl. 2009; 32:115–122. PMID: 17971166.

23. Enciso M, Muriel L, Fernández JL, Goyanes V, Segrelles E, Marcos M, et al. Infertile men with varicocele show a high relative proportion of sperm cells with intense nuclear damage level, evidenced by the sperm chromatin dispersion test. J Androl. 2006; 27:106–111. PMID: 16400086.

24. Talebi AR, Moein MR, Tabibnejad N, Ghasemzadeh J. Effect of varicocele on chromatin condensation and DNA integrityof ejaculated spermatozoa using cytochemical tests. Andrologia. 2008; 40:245–251. PMID: 18727735.
25. Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, Purvis K, et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod. 1999; 14:1039–1049. PMID: 10221239.

26. Sotolongo B, Huang TT, Isenberger E, Ward WS. An endogenous nuclease in hamster, mouse, and human spermatozoa cleaves DNA into loop-sized fragments. J Androl. 2005; 26:272–280. PMID: 15713834.

27. Fernández JL, Muriel L, Rivero MT, Goyanes V, Vazquez R, Alvarez JG. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl. 2003; 24:59–66. PMID: 12514084.
28. Fernández JL, Muriel L, Goyanes V, Segrelles E, Gosálvez J, Enciso M, et al. Simple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion test. Fertil Steril. 2005; 84:833–842. PMID: 16213830.
29. Sellami A, Chakroun N, Ben Zarrouk S, Sellami H, Kebaili S, Rebai T, et al. Assessment of chromatin maturity in human spermatozoa: useful aniline blue assay for routine diagnosis of male infertility. Adv Urol. 2013; DOI: 10.1155/2013/578631.

Fig. 1
Sperm viability test using eosin-nigrosin staining. Viable sperm heads were unstained and non-viable sperm heads were stained redpink (×400).
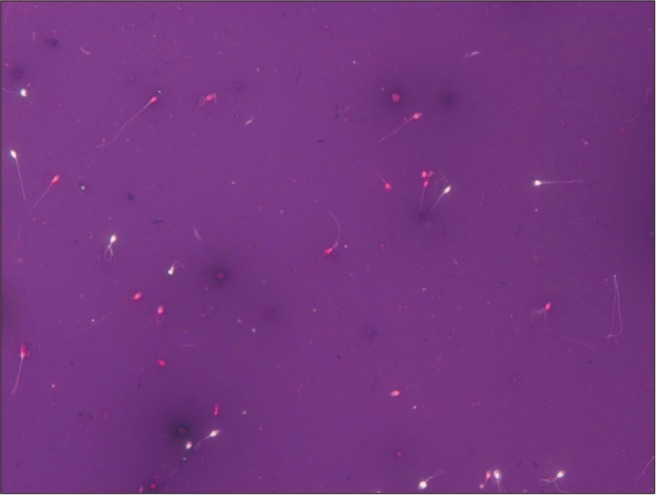
Fig. 3
Determination of sperm DNA fragmentation using the sperm chromatin dispersion test at magnifications of (A) ×200 and (B) ×400. For visualization, Diff-Quik staining was used. The arrows indicate a sperm without fragmentation (normal sperm) and the arrowheads indicate a sperm with fragmentation (DNA fragmented sperm).
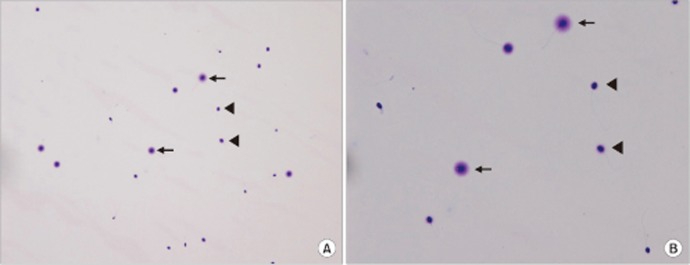
Table 1
Characteristics of sperm parameters and comparative proportions based on a 30% SDF threshold
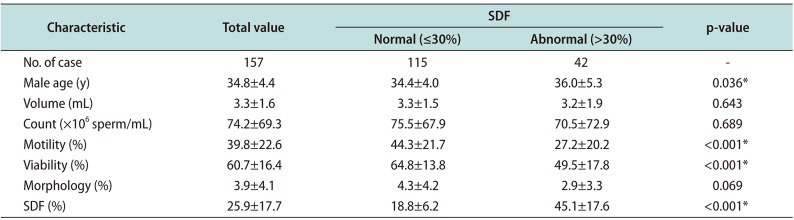
Table 2
Sperm parameters and DFI according to sperm count
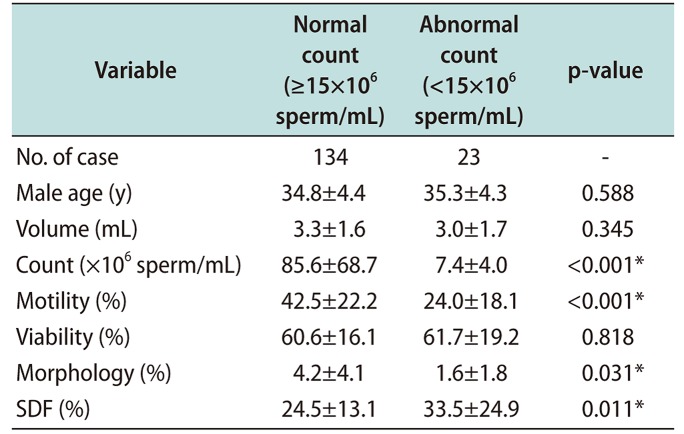
Table 3
Sperm parameters and DFI according to sperm motility
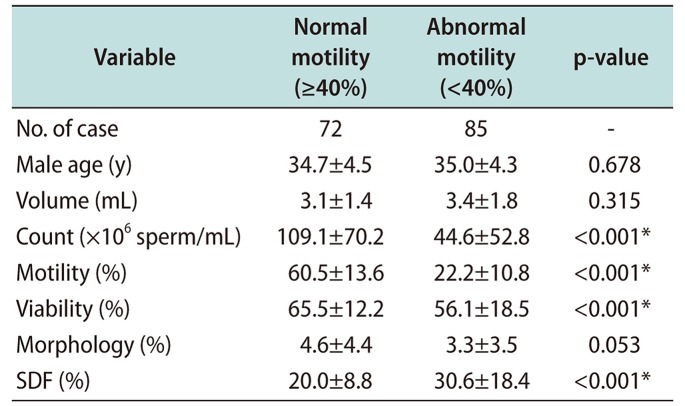
Table 4
Sperm parameters and DFI according to sperm viability
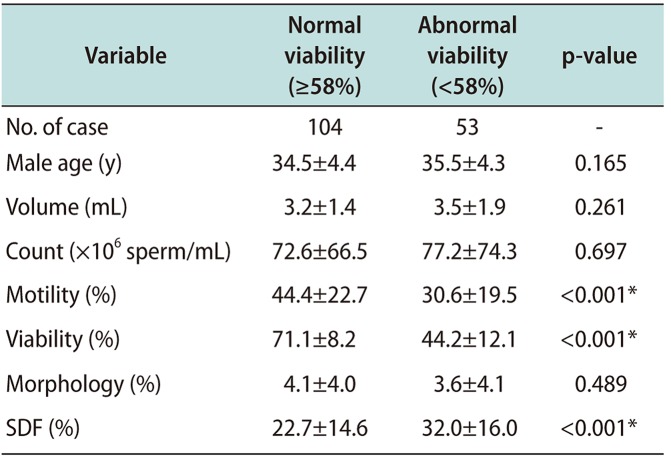
Table 5
Sperm parameters and DFI according to sperm morphology
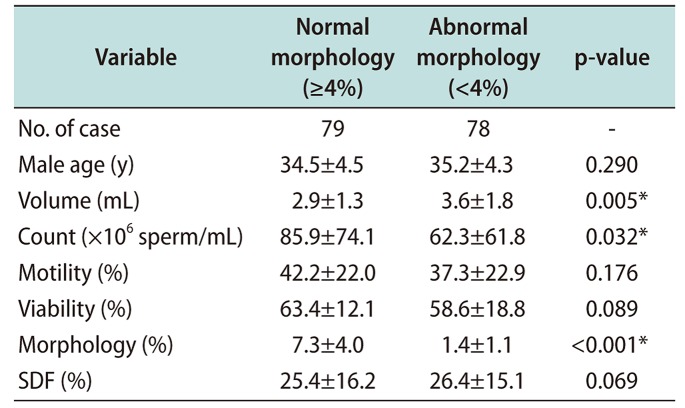
Table 6
Simple correlation coefficiency (r) among SDF and sperm parameters




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download