INTRODUCTION
1. Impact of metabolic syndrome on lower urinary tract symptoms
2. Bidirectional relationship between testosterone deficiency and metabolic syndrome
Table 1
The complex relationship between TD (hypogonadism) and Met S
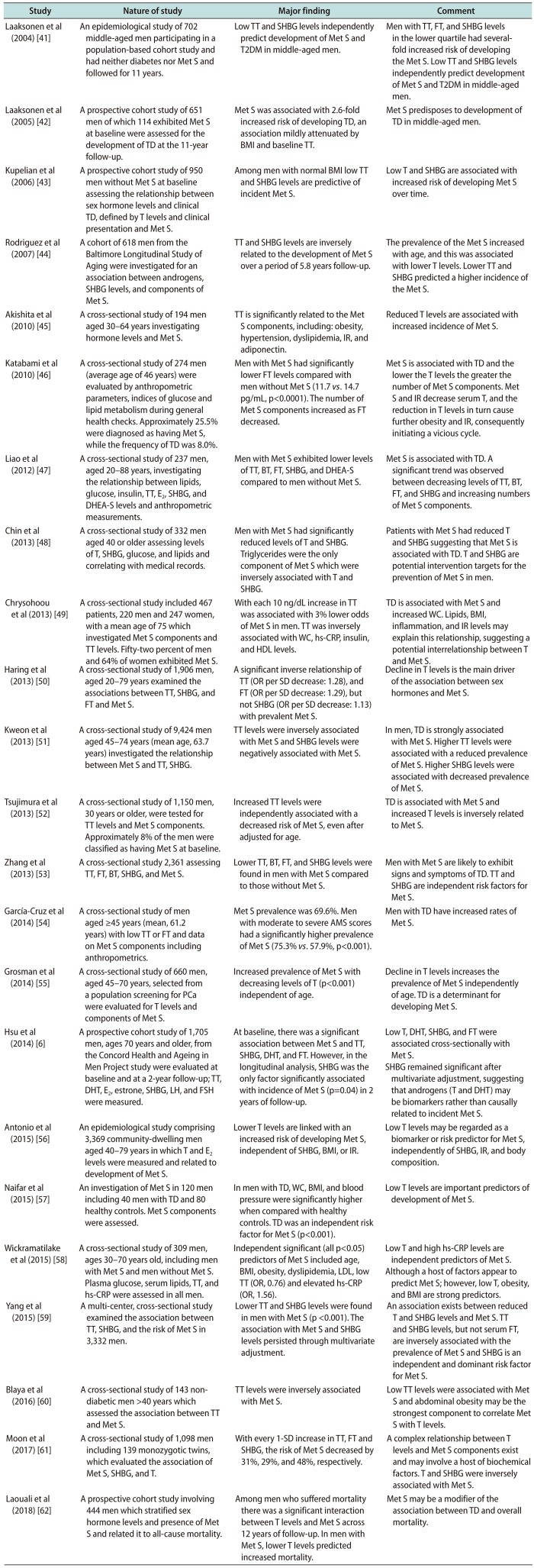
| Study | Nature of study | Major finding | Comment |
|---|---|---|---|
| Laaksonen et al (2004) [41] | An epidemiological study of 702 middle-aged men participating in a population-based cohort study and had neither diabetes nor Met S and followed for 11 years. | Low TT and SHBG levels independently predict development of Met S and T2DM in middle-aged men. | Men with TT, FT, and SHBG levels in the lower quartile had severalfold increased risk of developing the Met S. Low TT and SHBG levels independently predict development of Met S and T2DM in middle-aged men. |
| Laaksonen et al (2005) [42] | A prospective cohort study of 651 men of which 114 exhibited Met S at baseline were assessed for the development of TD at the 11-year follow-up. | Met S was associated with 2.6-fold increased risk of developing TD, an association mildly attenuated by BMI and baseline TT. | Met S predisposes to development of TD in middle-aged men. |
| Kupelian et al (2006) [43] | A prospective cohort study of 950 men without Met S at baseline assessing the relationship between sex hormone levels and clinical TD, defined by T levels and clinical presentation and Met S. | Among men with normal BMI low TT and SHBG levels are predictive of incident Met S. | Low T and SHBG are associated with increased risk of developing Met S over time. |
| Rodriguez et al (2007) [44] | A cohort of 618 men from the Baltimore Longitudinal Study of Aging were investigated for an association between androgens, SHBG levels, and components of Met S. | TT and SHBG levels are inversely related to the development of Met S over a period of 5.8 years follow-up. | The prevalence of the Met S increased with age, and this was associated with lower T levels. Lower TT and SHBG predicted a higher incidence of the Met S. |
| Akishita et al (2010) [45] | A cross-sectional study of 194 men aged 30–64 years investigating hormone levels and Met S. | TT is significantly related to the Met S components, including: obesity, hypertension, dyslipidemia, IR, and adiponectin. | Reduced T levels are associated with increased incidence of Met S. |
| Katabami et al (2010) [46] | A cross-sectional study of 274 men (average age of 46 years) were evaluated by anthropometric parameters, indices of glucose and lipid metabolism during general health checks. Approximately 25.5% were diagnosed as having Met S, while the frequency of TD was 8.0%. | Men with Met S had significantly lower FT levels compared with men without Met S (11.7 vs . 14.7 pg/mL, p<0.0001). The number of Met S components increased as FT decreased. | Met S is associated with TD and the lower the T levels the greater the number of Met S components. Met S and IR decrease serum T, and the reduction in T levels in turn cause further obesity and IR, consequently initiating a vicious cycle. |
| Liao et al (2012) [47] | A cross-sectional study of 237 men, aged 20–88 years, investigating the relationship between lipids, glucose, insulin, TT, E2, SHBG, and DHEA-S levels and anthropometric measurements. | Men with Met S exhibited lower levels of TT, BT, FT, SHBG, and DHEA-S compared to men without Met S. | Met S is associated with TD. A significant trend was observed between decreasing levels of TT, BT, FT, and SHBG and increasing numbers of Met S components. |
| Chin et al (2013) [48] | A cross-sectional study of 332 men aged 40 or older assessing levels of T, SHBG, glucose, and lipids and correlating with medical records. | Men with Met S had significantly reduced levels of T and SHBG. Triglycerides were the only component of Met S which were inversely associated with T and SHBG. | Patients with Met S had reduced T and SHBG suggesting that Met S is associated with TD. T and SHBG are potential intervention targets for the prevention of Met S in men. |
| Chrysohoou et al (2013) [49] | A cross-sectional study included 467 patients, 220 men and 247 women, with a mean age of 75 which investigated Met S components and TT levels. Fifty-two percent of men and 64% of women exhibited Met S. | With each 10 ng/dL increase in TT was associated with 3% lower odds of Met S in men. TT was inversely associated with WC, hs-CRP, insulin, and HDL levels. | TD is associated with Met S and increased WC. Lipids, BMI, inflammation, and IR levels may explain this relationship, suggesting a potential interrelationship between T and Met S. |
| Haring et al (2013) [50] | A cross-sectional study of 1,906 men, aged 20–79 years examined the associations between TT, SHBG, and FT and Met S. | A significant inverse relationship of TT (OR per SD decrease: 1.28), and FT (OR per SD decrease: 1.29), but not SHBG (OR per SD decrease: 1.13) with prevalent Met S. | Decline in T levels is the main driver of the association between sex hormones and Met S. |
| Kweon et al (2013) [51] | A cross-sectional study of 9,424 men aged 45–74 years (mean age, 63.7 years) investigated the relationship between Met S and TT, SHBG. | TT levels were inversely associated with Met S and SHBG levels were negatively associated with Met S. | In men, TD is strongly associated with Met S. Higher TT levels were associated with a reduced prevalence of Met S. Higher SHBG levels were associated with decreased prevalence of Met S. |
| Tsujimura et al (2013) [52] | A cross-sectional study of 1,150 men, 30 years or older, were tested for TT levels and Met S components. Approximately 8% of the men were classified as having Met S at baseline. | Increased TT levels were independently associated with a decreased risk of Met S, even after adjusted for age. | TD is associated with Met S and increased T levels is inversely related to Met S. |
| Zhang et al (2013) [53] | A cross-sectional study 2,361 assessing TT, FT, BT, SHBG, and Met S. | Lower TT, BT, FT, and SHBG levels were found in men with Met S compared to those without Met S. | Men with Met S are likely to exhibit signs and symptoms of TD. TT and SHBG are independent risk factors for Met S. |
| García-Cruz et al (2014) [54] | A cross-sectional study of men aged ≥45 years (mean, 61.2 years) with low TT or FT and data on Met S components including anthropometrics. | Met S prevalence was 69.6%. Men with moderate to severe AMS scores had a significantly higher prevalence of Met S (75.3% vs . 57.9%, p<0.001). | Men with TD have increased rates of Met S. |
| Grosman et al (2014) [55] | A cross-sectional study of 660 men, aged 45–70 years, selected from a population screening for Pca were evaluated for T levels and components of Met S. | Increased prevalence of Met S with decreasing levels of T (p<0.001) independent of age. | Decline in T levels increases the prevalence of Met S independently of age. TD is a determinant for developing Met S. |
| Hsu et al (2014) [6] | A prospective cohort study of 1,705 men, ages 70 years and older, from the Concord Health and Ageing in Men Project study were evaluated at baseline and at a 2-year follow-up; TT, DHT, E2, estrone, SHBG, LH, and FSH were measured. | At baseline, there was a significant association between Met S and TT, SHBG, DHT, and FT. However, in the longitudinal analysis, SHBG was the only factor significantly associated with incidence of Met S (p=0.04) in 2 years of follow-up. | Low T, DHT, SHBG, and FT were associated cross-sectionally with Met S. |
| SHBG remained significant after multivariate adjustment, suggesting that androgens (T and DHT) may be biomarkers rather than causally related to incident Met S. | |||
| Antonio et al (2015) [56] | An epidemiological study comprising 3,369 community-dwelling men aged 40–79 years in which T and E2 levels were measured and related to development of Met S. | Lower T levels are linked with an increased risk of developing Met S, independent of SHBG, BMI, or IR. | Low T levels may be regarded as a biomarker or risk predictor for Met S, independently of SHBG, IR, and body composition. |
| Naifar et al (2015) [57] | An investigation of Met S in 120 men including 40 men with TD and 80 healthy controls. Met S components were assessed. | In men with TD, WC, BMI, and blood pressure were significantly higher when compared with healthy controls. TD was an independent risk factor for Met S (p<0.001). | Low T levels are important predictors of development of Met S. |
| Wickramatilake et al (2015) [58] | A cross-sectional study of 309 men, ages 30–70 years old, including men with Met S and men without Met S. Plasma glucose, serum lipids, TT, and hs-CRP were assessed in all men. | Independent significant (all p<0.05) predictors of Met S included age, BMI, obesity, dyslipidemia, LDL, low TT (OR, 0.76) and elevated hs-CRP (OR, 1.56). | Low T and high hs-CRP levels are independent predictors of Met S. |
| Although a host of factors appear to predict Met S; however, low T, obesity, and BMI are strong predictors. | |||
| Yang et al (2015) [59] | A multi-center, cross-sectional study examined the association between TT, SHBG, and the risk of Met S in 3,332 men. | Lower TT and SHBG levels were found in men with Met S (p <0.001). The association with Met S and SHBG levels persisted through multivariate adjustment. | An association exists between reduced T and SHBG levels and Met S. TT and SHBG levels, but not serum FT, are inversely associated with the prevalence of Met S and SHBG is an independent and dominant risk factor for Met S. |
| Blaya et al (2016) [60] | A cross-sectional study of 143 nondiabetic men >40 years which assessed the association between TT and Met S. | TT levels were inversely associated with Met S. | Low TT levels were associated with Met S and abdominal obesity may be the strongest component to correlate Met S with T levels. |
| Moon et al (2017) [61] | A cross-sectional study of 1,098 men including 139 monozygotic twins, which evaluated the association of Met S, SHBG, and T. | With every 1-SD increase in TT, FT and SHBG, the risk of Met S decreased by 31%, 29%, and 48%, respectively. | A complex relationship between T levels and Met S components exist and may involve a host of biochemical factors. T and SHBG were inversely associated with Met S. |
| Laouali et al (2018) [62] | A prospective cohort study involving 444 men which stratified sex hormone levels and presence of Met S and related it to all-cause mortality | Among men who suffered mortality there was a significant interaction between T levels and Met S across 12 years of follow-up. In men with Met S, lower T levels predicted increased mortality. | Met S may be a modifier of the association between TD and overall mortality. |
TD: testosterone deficiency, Met S: metabolic syndrome, T: testosterone, SHBG: sex-hormone binding globulin, TT: total testosterone, E2: estradiol, DHEA-S: dehydroepiandrosterone sulfate, FT: free testosterone, BT: bioavailable testosterone, PCa: prostate cancer, DHT: dihydrotestosterone, LH: luteinizing hormone, FSH: follicle stimulating hormone, hs-CRP: high-sensitivity C-reactive protein, T2DM: type 2 diabetes mellitus, BMI: body mass index, IR: insulin resistance, WC: waist circumference, HDL: high-density lipoprotein, OR: odds ratio, SD: standard deviation, AMS: aging males' symptom, LDL: low-density lipoprotein.
METABOLIC SYNDROME, TESTOSTERONE DEFICIENCY, AND PATHOPHYSIOLOGY OF LOWER URINARY TRACT SYMPTOMS
Table 2
The complex relationship between TD, Met S, and LUTS
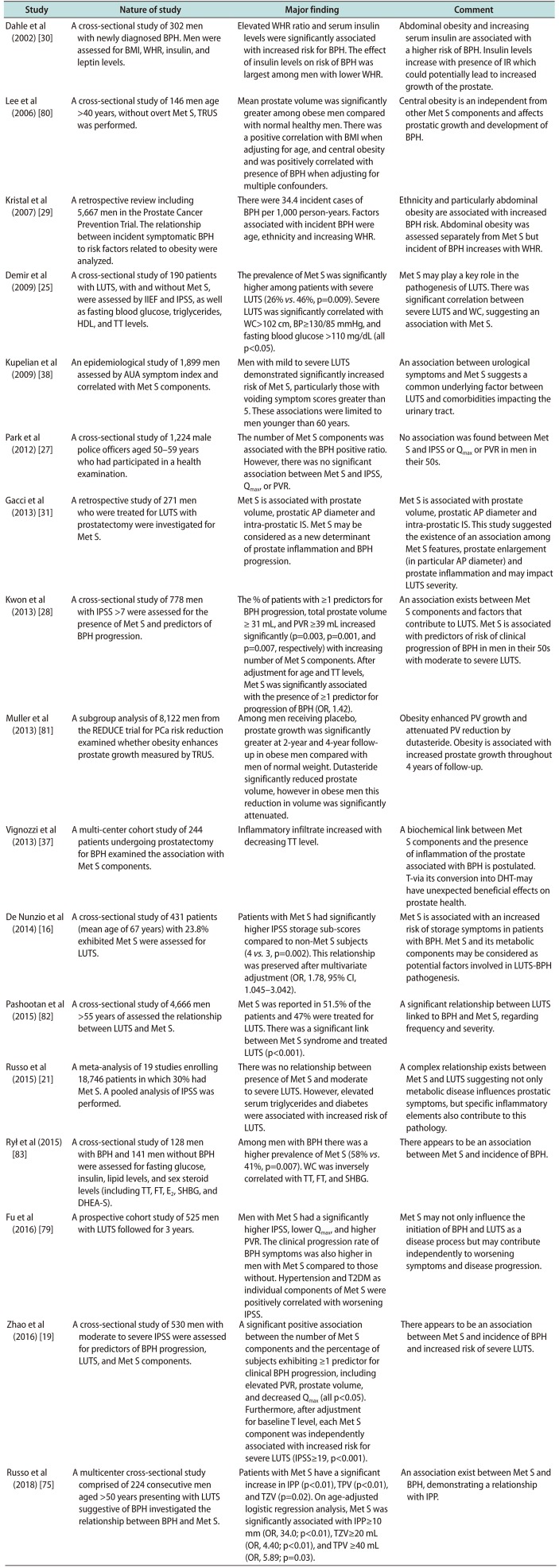
| Study | Nature of study | Major finding | Comment |
|---|---|---|---|
| Dahle et al (2002) [30] | A cross-sectional study of 302 men with newly diagnosed BPH. Men were assessed for BMI, WHR, insulin, and leptin levels. | Elevated WHR ratio and serum insulin levels were significantly associated with increased risk for BPH. The effect of insulin levels on risk of BPH was largest among men with lower WHR. | Abdominal obesity and increasing serum insulin are associated with a higher risk of BPH. Insulin levels increase with presence of IR which could potentially lead to increased growth of the prostate. |
| Lee et al (2006) [80] | A cross-sectional study of 146 men age >40 years, without overt Met S, TRUS was performed. | Mean prostate volume was significantly greater among obese men compared with normal healthy men. There was a positive correlation with BMI when adjusting for age, and central obesity and was positively correlated with presence of BPH when adjusting for multiple confounders. | Central obesity is an independent from other Met S components and affects prostatic growth and development of BPH. |
| Kristal et al (2007) [29] | A retrospective review including 5,667 men in the Prostate Cancer Prevention Trial. The relationship between incident symptomatic BPH to risk factors related to obesity were analyzed. | There were 34.4 incident cases of BPH per 1,000 person-years. Factors associated with incident BPH were age, ethnicity and increasing WHR. | Ethnicity and particularly abdominal obesity are associated with increased BPH risk. Abdominal obesity was assessed separately from Met S but incident of BPH increases with WHR. |
| Demir et al (2009) [25] | A cross-sectional study of 190 patients with LUTS, with and without Met S, were assessed by IIEF and IPSS, as well as fasting blood glucose, triglycerides, HDL, and TT levels. | The prevalence of Met S was significantly higher among patients with severe LUTS (26% vs. 46%, p=0.009). Severe LUTS was significantly correlated with WC>102 cm, BP≥130/85 mmHg, and fasting blood glucose >110 mg/dL (all p<0.05). | Met S may play a key role in the pathogenesis of LUTS. There was significant correlation between severe LUTS and WC, suggesting an association with Met S. |
| Kupelian et al (2009) [38] | An epidemiological study of 1,899 men assessed by AUA symptom index and correlated with Met S components. | Men with mild to severe LUTS demonstrated significantly increased risk of Met S, particularly those with voiding symptom scores greater than 5. These associations were limited to men younger than 60 years. | An association between urological symptoms and Met S suggests a common underlying factor between LUTS and comorbidities impacting the urinary tract. |
| Park et al (2012) [27] | A cross-sectional study of 1,224 male police officers aged 50–59 years who had participated in a health examination. | The number of Met S components was associated with the BPH positive ratio. However, there was no significant association between Met S and IPSS, Qmax, or PVR. | No association was found between Met S and IPSS or Qmax or PVR in men in their 50s. |
| Gacci et al (2013) [31] | A retrospective study of 271 men who were treated for LUTS with prostatectomy were investigated for Met S. | Met S is associated with prostate volume, prostatic AP diameter and intra-prostatic IS. Met S may be considered as a new determinant of prostate inflammation and BPH progression. | Met S is associated with prostate volume, prostatic AP diameter and intra-prostatic IS. This study suggested the existence of an association among Met S features, prostate enlargement (in particular AP diameter) and prostate inflammation and may impact LUTS severity. |
| Kwon et al (2013) [28] | A cross-sectional study of 778 men with IPSS >7 were assessed for the presence of Met S and predictors of BPH progression. | The % of patients with ≥1 predictors for BPH progression, total prostate volume ≥ 31 mL, and PVR ≥39 mL increased significantly (p=0.003, p=0.001, and p=0.007, respectively) with increasing number of Met S components. After adjustment for age and TT levels, Met S was significantly associated with the presence of ≥1 predictor for progression of BPH (OR, 1.42). | An association exists between Met S components and factors that contribute to LUTS. Met S is associated with predictors of risk of clinical progression of BPH in men in their 50s with moderate to severe LUTS. |
| Muller et al (2013) [81] | A subgroup analysis of 8,122 men from the REDUCE trial for PCa risk reduction examined whether obesity enhances prostate growth measured by TRUS. | Among men receiving placebo, prostate growth was significantly greater at 2-year and 4-year followup in obese men compared with men of normal weight. Dutasteride significantly reduced prostate volume, however in obese men this reduction in volume was significantly attenuated. | Obesity enhanced PV growth and attenuated PV reduction by dutasteride. Obesity is associated with increased prostate growth throughout 4 years of follow-up. |
| Vignozzi et al (2013) [37] | A multi-center cohort study of 244 patients undergoing prostatectomy for BPH examined the association with Met S components. | Inflammatory infiltrate increased with decreasing TT level. | A biochemical link between Met S components and the presence of inflammation of the prostate associated with BPH is postulated. T-via its conversion into DHT-may have unexpected beneficial effects on prostate health. |
| De Nunzio et al (2014) [16] | A cross-sectional study of 431 patients (mean age of 67 years) with 23.8% exhibited Met S were assessed for LUTS. | Patients with Met S had significantly higher IPSS storage sub-scores compared to non-Met S subjects (4 vs. 3, p=0.002). This relationship was preserved after multivariate adjustment (OR, 1.78, 95% CI, 1.045–3.042). | Met S is associated with an increased risk of storage symptoms in patients with BPH. Met S and its metabolic components may be considered as potential factors involved in LUTS-BPH pathogenesis. |
| Pashootan et al (2015) [82] | A cross-sectional study of 4,666 men >55 years of assessed the relationship between LUTS and Met S. | Met S was reported in 51.5% of the patients and 47% were treated for LUTS. There was a significant link between Met S syndrome and treated LUTS (p<0.001). | A significant relationship between LUTS linked to BPH and Met S, regarding frequency and severity. |
| Russo et al (2015) [21] | A meta-analysis of 19 studies enrolling 18,746 patients in which 30% had Met S. A pooled analysis of IPSS was performed. | There was no relationship between presence of Met S and moderate to severe LUTS. However, elevated serum triglycerides and diabetes were associated with increased risk of LUTS. | A complex relationship exists between Met S and LUTS suggesting not only metabolic disease influences prostatic symptoms, but specific inflammatory elements also contribute to this pathology. |
| Rył et al (2015) [83] | A cross-sectional study of 128 men with BPH and 141 men without BPH were assessed for fasting glucose, insulin, lipid levels, and sex steroid levels (including TT, FT, E2, SHBG, and DHEA-S). | Among men with BPH there was a higher prevalence of Met S (58% vs. 41%, p=0.007). WC was inversely correlated with TT, FT, and SHBG. | There appears to be an association between Met S and incidence of BPH. |
| Fu et al (2016) [79] | A prospective cohort study of 525 men with LUTS followed for 3 years. | Men with Met S had a significantly higher IPSS, lower Qmax, and higher PVR. The clinical progression rate of BPH symptoms was also higher in men with Met S compared to those without. Hypertension and T2DM as individual components of Met S were positively correlated with worsening IPSS. | Met S may not only influence the initiation of BPH and LUTS as a disease process but may contribute independently to worsening symptoms and disease progression. |
| Zhao et al (2016) [19] | A cross-sectional study of 530 men with moderate to severe IPSS were assessed for predictors of BPH progression, LUTS, and Met S components. | A significant positive association between the number of Met S components and the percentage of subjects exhibiting ≥1 predictor for clinical BPH progression, including elevated PVR, prostate volume, and decreased Qmax (all p<0.05). Furthermore, after adjustment for baseline T level, each Met S component was independently associated with increased risk for severe LUTS (IPSS≥19, p<0.001). | There appears to be an association between Met S and incidence of BPH and increased risk of severe LUTS. |
| Russo et al (2018) [75] | A multicenter cross-sectional study comprised of 224 consecutive men aged >50 years presenting with LUTS suggestive of BPH investigated the relationship between BPH and Met S. | Patients with Met S have a significant increase in IPP (p<0.01), TPV (p<0.01), and TZV (p=0.02). On age-adjusted logistic regression analysis, Met S was significantly associated with IPP≥10 mm (OR, 34.0; p<0.01), TZV≥20 mL (OR, 4.40; p<0.01), and TPV ≥40 mL (OR, 5.89; p=0.03). | An association exist between Met S and BPH, demonstrating a relationship with IPP. |
TD: testosterone deficiency, Met S: metabolic syndrome, LUTS: lower urinary tract symptoms, BPH: benign prostatic hypertrophy, BMI: body mass index, WHR: waist-to-hip ratio, TRUS: transrectal ultrasound, IIEF: international index of erectile function, IPSS: International Prostate Symptom Score, HDL: high-density lipoprotein, TT: total testosterone, AUA: American Urological Association, PCa: prostate cancer, FT: free testosterone, E2: estradiol, SHBG: sex-hormone binding globulin, DHEA-S: dehydroepiandrosterone sulfate, WC: waist circumference, BP: blood pressure, Qmax: maximum urinary flow rate, PVR: post-voiding residual volume, AP: anteriorposterior, IS: inflammatory score, OR: odds ratio, CI: confidence interval, T2DM: type 2 diabetes mellitus, T: testosterone, IPP: intravesical prostatic protrusion, TPV: total prostate volume, TZV: transitional zone volume, IR: insulin resistance, PV: prostate volume, DHT: dihydrotestosterone.
PATHOPHYSIOLOGICAL MECHANISMS OF LOWER URINARY TRACT SYMPTOMS
DOES TESTOSTERONE THERAPY IMPROVES LOWER URINARY TRACT SYMPTOMS?
1. Testosterone therapy ameliorates metabolic syndrome components
Table 3
T therapy ameliorates components of Met S

| Study | Nature of study | Major finding | Comment |
|---|---|---|---|
| Saad et al (2008) [116] | This was a 9-month observational study of men with hypogonadism who were treated with either IM with TU 1,000 mg every 12 weeks (mean age, 60 years) or T gel 50 mg daily (mean age, 61 years). | T therapy resulted in reduction WC concomitant with changes in total cholesterol, LDL, and HDL. Furthermore, in men treated with TU, SHBG levels increased, while T gel was associated with a reduction in SHBG levels. | T therapy improves Met S components. |
| Heufelder et al (2009) [117] | Randomized control trial of 32 hypogonadal men with Met S and newly diagnosed T2DM were treated with diet and exercise alone (n=16) or in combination with transdermal T 50 mg (n=16). Patients were followed for 52 weeks for assessment of Met S components, TD, and diabetes. | T therapy significantly improved fasting glucose, HbA1c, triglycerides insulin sensitivity and reduced hs-CRP levels and WC. All patients on T therapy achieved goal HbA1c of <7.0%, and 87.5% achieved HbA1c<6.5%. | T therapy improves WC, HbA1c, glucose, and triglyceride levels and reduces hs-CRP, suggesting improvement in Met S. |
| Aversa et al (2010) [118] | Fifty patients with Met S were randomized to receive IM TU (1,000 mg every 12 weeks) or placebo gel daily for 24 months. Outcomes included HOMA-IR, carotid intima-media thickness, and hs-CRP. | At 24 months, there was an absolute reduction in the prevalence of Met S of 65% in subjects randomized to TU. The primary changes in Met S components occurred in WC, visceral fat mass, and HOMA-IR (all p<0.0001). | T therapy ameliorates Met S components and reduces IR and reduces WC and fat mass. |
| Haider et al (2010) [119] | An observational study comprised of 122 men with TD (mean age, 59.6 years) were treated with IM TU for 24 months and assessed for elements of Met S and anthropometric parameters. | A decrease over the study period was observed in BMI and WC. At baseline 38.5% of subjects met criteria for Met S, while only 9.0% met these criteria by study end. | T therapy ameliorates Met S and reduces BMI and WC. |
| Kalinchenko et al (2010) [120] | A randomized, placebo-controlled, phase 3 trial in which 184 men with Met S and TD were evaluated. Subjects were randomized to receive either IM TU (1,000 mg) or placebo at baseline, 6 weeks, and 18 weeks. Follow-up was for 30 weeks. Outcomes included BMI, WC, WHR, insulin, leptin, lipids, CRP, and several inflammatory markers. | T therapy was associated with significant decreases in weight, BMI, WC, leptin, and insulin. There were no significant changes in lipid profile. Additionally, decreased levels of IL-1β, TNF-α, and CRP were observed. | T therapy reduces weight, BMI, and WC and improves insulin sensitivity and attenuates inflammatory cytokines. |
| Bhattacharya et al (2011) [121] | In this observational study, 849 men from the Testim® Registry in the United States who were prescribed 1% testosterone gel were assessed for hormonal milieu and Met S components. | The 37% of patients had Met S at baseline. Among patients with Met S there were significantly lower TT and SHBG levels (p<0.0001, p=0.01, respectively). Patients within the lowest quartile of TT (<206 ng/ dL) had a significantly increased risk of Met S compared to the highest quartile (≥331 ng/dL) (OR=2.66, 95% CI, 1.60–4.43). After 12 months of T therapy, Met S patients demonstrated improved WC, fasting glucose, and BP. Lipid measures were unaffected in both groups. | Hypogonadal patients with Met S had on average lower baseline TT levels and a higher number of comorbidities. Because T therapy resulted in improved Met S parameters, the authors suggest there may be a role for measuring T levels in men with Met S. |
| Jeong et al (2011) [122] | This was a retrospective review of 200 men diagnosed with TD and undergoing T therapy. Patients were divided into two groups, 71 men with Met S and 129 without Met S. | Among men with Met S, T therapy was associated with improved fasting glucose and WC. Among men without Met S, T therapy was associated with improved WC, BMI, total cholesterol, LDL, and fasting glucose. In both groups of men, T therapy was associated with improved AMS and IIEF scores. | T therapy improves fasting glucose and reduces WC and BMI concomitant with reduction in LDL and total cholesterol. |
| Jones et al (2011) [123] | 220 hypogonadal men with T2DM and/ or Met S were enrolled in a randomized, double-blind, placebo-controlled study to assess the efficacy and safety of 2% transdermal T gel over 12 months. The primary outcome was change in HOMA-IR, while secondary outcomes included body composition, glycemic control, lipid levels, and sexual function. No medication changes were allowed in months 0–6, while they were allowed in months 7–12. | T therapy reduced HOMA-IR measures by 15.2% at 6 months and 16.4% at 12 months (p=0.018, 0.006, respectively). Among men with T2DM, T therapy resulted in significantly better glycemic control as measured by HbA1c (−0.446%, p=0.035). There were no differences between groups in terms of serious adverse events. | T therapy improves insulin sensitivity and reduces HOMA-IR. |
| Hoyos et al (2012) [124] | This study was an 18-week randomized, double-blind, placebo-controlled trial of 67 men. Men in the experimental group were given 1,000 mg of IM TU and placed on a low-calorie diet. | Experimental group had increased insulin sensitivity (−1.14 units, p<0.05), reduced liver fat educed liver fat (0.09 Hounsfield attenuation ratio, p=0.03) and increased muscle mass (1.6 kg, p=0.0009) compared to placebo. Additionally, testosterone decreased arterial stiffness in the experimental group by 3.2%. | Although across the 18 weeks, components of Met S were ameliorated, the overall prevalence of Met S remained the same, regardless of T therapy. |
| Stanworth et al (2013) [125] | 139 men with hypogonadism and T2DM, 73 of whom received 2% transdermal T gel, were assessed in a subgroup analysis from a larger randomized, placebo-controlled trial (TIMES2 study). The main outcome measure was the regression coefficient of androgen receptor CAG polymorphism from linear regression models for multiple covariates. | The presence of androgen receptor CAG polymorphism as associated with changes in fasting insulin, triglycerides, and diastolic blood pressure in response to T therapy. There were further trends to association with HOMA-IR and PSA levels, but these were non-significant. | The authors posit that the presence of androgen receptor CAG polymorphism may modulate the response to T therapy in men with regards to several, but not all, Met S and diabetes related variables. |
| Francomano et al (2014) [126] | 20 hypogonadal men with Met S (mean age, 58 years) were treated with IM TU every 12 weeks for 5 years. Twenty matched controls were also identified, and primary endpoints were variations in hormonal and metabolic parameters. | Among men who received T therapy, significant reductions in WC (p<0.0001), weight (−15 kg, p<0.0001), and HbA1c (−1.6%, p<0.0001) were observed. Additionally, HOMA-IR, lipid profile, and BP were all improved significantly compared to controls. | T therapy improves body composition and reduces weight and improves hyperglycemia and insulin sensitivity and dyslipidemia. |
| Traish et al (2014) [127] | This was a retrospective study of 255 men (mean age, 58 years) with below normal TT levels (mean, 9.93 nmol/L) including at least mild TD symptoms assessed by AMS score. All subjects received IM TU 1,000 mg at baseline, 6 weeks, and every 3 months thereafter for up to 5 years. | T therapy resulted in changes in total cholesterol (−93.46 mg/dL), LDL (−53.95 mg/dL), triglycerides (−86.38 mg/dL), and HDL (+2.68 mg/dL). There were additionally reductions in systolic and diastolic BP, HbA1c, CRP level, and liver function enzymes. | T therapy ameliorates Met S components in men with TD. |
| Yassin et al (2014) [128] | This was an observational study in which 261 men (mean age, 59.5 years) with hypogonadism and ED were treated with IM TU 1,000 mg at baseline, 6 weeks, and subsequently every 3 months. Primary outcome measures included Met S and obesity parameters, lipids, glucose and HbA1c, BP, and health-related QoL. | T therapy was associated with significant improvement in body weight, WC, BMI, and cholesterol. Additionally, fasting glucose, HbA1c, and BP were significantly reduced over the 5-year follow-up period. T therapy also improved erectile function and health-related QoL. | T therapy improves WC, BMI and lipid profiles and reduces glucose and HbA1c in men with TD. |
| Shigehara et al (2018) [129] | A randomized controlled study including 65 hypogonadal men with Met S. The T therapy group (n=32) was administered 250 mg of TE as an intramuscular injection every 4 week for 1 year. Met S components including lipid levels and HbA1c were evaluated. | T therapy produced significant improvements in WC, body fat percentage, and triglycerides at 12-month follow-up (all p<0.05), all of which were significantly different from changes in the control group. | T therapy in men with TD improves WC and body composition. |
T: testosterone, Met S: metabolic syndrome, IM: intramuscular, TU: testosterone undecanoate, T2DM: type 2 diabetes mellitus, TD: testosterone deficiency, HOMA-IR: homeostasis model assessment of insulin resistance, hs-CRP: high-sensitivity C-reactive protein, BMI: body mass index, WC: waist circumference, WHR: waist-to-hip ratio, TT: total testosterone, AMS: aging males' symptom, ED: erectile dysfunction, HbA1c: hemoglobin A1c, BP: blood pressure, QoL: quality of life, TE: testosterone enanthate, LDL: low-density lipoprotein, HDL: high-density lipoprotein, SHBG: sex-hormone binding globulin, IL: interleukin, TNF: tumor necrosis factor, OR: odds ratio, CI: confidence interval, IIEF: international index of erectile function, PSA: prostate specific antigen.
2. Testosterone therapy and lower urinary tract symptoms
Table 4
T therapy improves LUTS in men with TD and Met S

| Study | Nature of study | Major finding | Comment |
|---|---|---|---|
| Saad et al (2008) [116] | This was a 9-month observational study of men with hypogonadism who were treated with either IM with TU 1,000 mg every 12 weeks (mean age, 60 years) or T gel 50 mg daily (mean age, 61 years). | T therapy resulted in reduction WC concomitant with changes in total cholesterol, LDL, and HDL. Furthermore, in men treated with TU, SHBG levels increased, while T gel was associated with a reduction in SHBG levels. | T therapy improves Met S components. |
| Heufelder et al (2009) [117] | Randomized control trial of 32 hypogonadal men with Met S and newly diagnosed T2DM were treated with diet and exercise alone (n=16) or in combination with transdermal T 50 mg (n=16). Patients were followed for 52 weeks for assessment of Met S components, TD, and diabetes. | T therapy significantly improved fasting glucose, HbA1c, triglycerides insulin sensitivity and reduced hs-CRP levels and WC. All patients on T therapy achieved goal HbA1c of <7.0%, and 87.5% achieved HbA1c<6.5%. | T therapy improves WC, HbA1c, glucose, and triglyceride levels and reduces hs-CRP, suggesting improvement in Met S. |
| Aversa et al (2010) [118] | Fifty patients with Met S were randomized to receive IM TU (1,000 mg every 12 weeks) or placebo gel daily for 24 months. Outcomes included HOMA-IR, carotid intima-media thickness, and hs-CRP. | At 24 months, there was an absolute reduction in the prevalence of Met S of 65% in subjects randomized to TU. The primary changes in Met S components occurred in WC, visceral fat mass, and HOMA-IR (all p<0.0001). | T therapy ameliorates Met S components and reduces IR and reduces WC and fat mass. |
| Haider et al (2010) [119] | An observational study comprised of 122 men with TD (mean age, 59.6 years) were treated with IM TU for 24 months and assessed for elements of Met S and anthropometric parameters. | A decrease over the study period was observed in BMI and WC. At baseline 38.5% of subjects met criteria for Met S, while only 9.0% met these criteria by study end. | T therapy ameliorates Met S and reduces BMI and WC. |
| Kalinchenko et al (2010) [120] | A randomized, placebo-controlled, phase 3 trial in which 184 men with Met S and TD were evaluated. Subjects were randomized to receive either IM TU (1,000 mg) or placebo at baseline, 6 weeks, and 18 weeks. Follow-up was for 30 weeks. Outcomes included BMI, WC, WHR, insulin, leptin, lipids, CRP, and several inflammatory markers. | T therapy was associated with significant decreases in weight, BMI, WC, leptin, and insulin. There were no significant changes in lipid profile. Additionally, decreased levels of IL-1β, TNF-α, and CRP were observed. | T therapy reduces weight, BMI, and WC and improves insulin sensitivity and attenuates inflammatory cytokines. |
| Bhattacharya et al (2011) [121] | In this observational study, 849 men from the Testim® Registry in the United States who were prescribed 1% testosterone gel were assessed for hormonal milieu and Met S components. | The 37% of patients had Met S at baseline. Among patients with Met S there were significantly lower TT and SHBG levels (p<0.0001, p=0.01, respectively). Patients within the lowest quartile of TT (<206 ng/ dL) had a significantly increased risk of Met S compared to the highest quartile (≥331 ng/dL) (OR=2.66, 95% CI, 1.60–4.43). After 12 months of T therapy, Met S patients demonstrated improved WC, fasting glucose, and BP. Lipid measures were unaffected in both groups. | Hypogonadal patients with Met S had on average lower baseline TT levels and a higher number of comorbidities. Because T therapy resulted in improved Met S parameters, the authors suggest there may be a role for measuring T levels in men with Met S. |
| Jeong et al (2011) [122] | This was a retrospective review of 200 men diagnosed with TD and undergoing T therapy. Patients were divided into two groups, 71 men with Met S and 129 without Met S. | Among men with Met S, T therapy was associated with improved fasting glucose and WC. Among men without Met S, T therapy was associated with improved WC, BMI, total cholesterol, LDL, and fasting glucose. In both groups of men, T therapy was associated with improved AMS and IIEF scores. | T therapy improves fasting glucose and reduces WC and BMI concomitant with reduction in LDL and total cholesterol. |
| Jones et al (2011) [123] | 220 hypogonadal men with T2DM and/ or Met S were enrolled in a randomized, double-blind, placebo-controlled study to assess the efficacy and safety of 2% transdermal T gel over 12 months. The primary outcome was change in HOMA-IR, while secondary outcomes included body composition, glycemic control, lipid levels, and sexual function. No medication changes were allowed in months 0–6, while they were allowed in months 7–12. | T therapy reduced HOMA-IR measures by 15.2% at 6 months and 16.4% at 12 months (p=0.018, 0.006, respectively). Among men with T2DM, T therapy resulted in significantly better glycemic control as measured by HbA1c (−0.446%, p=0.035). There were no differences between groups in terms of serious adverse events. | T therapy improves insulin sensitivity and reduces HOMA-IR. |
| Hoyos et al (2012) [124] | This study was an 18-week randomized, double-blind, placebo-controlled trial of 67 men. Men in the experimental group were given 1,000 mg of IM TU and placed on a low-calorie diet. | Experimental group had increased insulin sensitivity (−1.14 units, p<0.05), reduced liver fat educed liver fat (0.09 Hounsfield attenuation ratio, p=0.03) and increased muscle mass (1.6 kg, p=0.0009) compared to placebo. Additionally, testosterone decreased arterial stiffness in the experimental group by 3.2%. | Although across the 18 weeks, components of Met S were ameliorated, the overall prevalence of Met S remained the same, regardless of T therapy. |
| Stanworth et al (2013) [125] | 139 men with hypogonadism and T2DM, 73 of whom received 2% transdermal T gel, were assessed in a subgroup analysis from a larger randomized, placebo-controlled trial (TIMES2 study). The main outcome measure was the regression coefficient of androgen receptor CAG polymorphism from linear regression models for multiple covariates. | The presence of androgen receptor CAG polymorphism as associated with changes in fasting insulin, triglycerides, and diastolic blood pressure in response to T therapy. There were further trends to association with HOMA-IR and PSA levels, but these were non-significant. | The authors posit that the presence of androgen receptor CAG polymorphism may modulate the response to T therapy in men with regards to several, but not all, Met S and diabetes related variables. |
| Francomano et al (2014) [126] | 20 hypogonadal men with Met S (mean age, 58 years) were treated with IM TU every 12 weeks for 5 years. Twenty matched controls were also identified, and primary endpoints were variations in hormonal and metabolic parameters. | Among men who received T therapy, significant reductions in WC (p<0.0001), weight (−15 kg, p<0.0001), and HbA1c (−1.6%, p<0.0001) were observed. Additionally, HOMA-IR, lipid profile, and BP were all improved significantly compared to controls. | T therapy improves body composition and reduces weight and improves hyperglycemia and insulin sensitivity and dyslipidemia. |
| Traish et al (2014) [127] | This was a retrospective study of 255 men (mean age, 58 years) with below normal TT levels (mean, 9.93 nmol/L) including at least mild TD symptoms assessed by AMS score. All subjects received IM TU 1,000 mg at baseline, 6 weeks, and every 3 months thereafter for up to 5 years. | T therapy resulted in changes in total cholesterol (−93.46 mg/dL), LDL (−53.95 mg/dL), triglycerides (−86.38 mg/dL), and HDL (+2.68 mg/dL). There were additionally reductions in systolic and diastolic BP, HbA1c, CRP level, and liver function enzymes. | T therapy ameliorates Met S components in men with TD. |
| Yassin et al (2014) [128] | This was an observational study in which 261 men (mean age, 59.5 years) with hypogonadism and ED were treated with IM TU 1,000 mg at baseline, 6 weeks, and subsequently every 3 months. Primary outcome measures included Met S and obesity parameters, lipids, glucose and HbA1c, BP, and health-related QoL. | T therapy was associated with significant improvement in body weight, WC, BMI, and cholesterol. Additionally, fasting glucose, HbA1c, and BP were significantly reduced over the 5-year follow-up period. T therapy also improved erectile function and health-related QoL. | T therapy improves WC, BMI and lipid profiles and reduces glucose and HbA1c in men with TD. |
| Shigehara et al (2018) [129] | A randomized controlled study including 65 hypogonadal men with Met S. The T therapy group (n=32) was administered 250 mg of TE as an intramuscular injection every 4 week for 1 year. Met S components including lipid levels and HbA1c were evaluated. | T therapy produced significant improvements in WC, body fat percentage, and triglycerides at 12-month follow-up (all p<0.05), all of which were significantly different from changes in the control group. | T therapy in men with TD improves WC and body composition. |
T: testosterone, LUTS: lower urinary tract symptoms, TD: testosterone deficiency, Met S: metabolic syndrome, IM: intramuscular, TU: testosterone undecanoate, GL: Glowmin, IPSS: International Prostate Symptom Score, AMS: aging males' symptom, IIEF: international index of erectile function, Qmax: maximum urinary flow rate, FT: free testosterone, PVR: post-voiding residual volume, WC: waist circumference, LDL: low-density lipoprotein, HbA1c: hemoglobin A1c, CRP: C-reactive protein.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download