Abstract
Aging affects metabolism, leading to physiological and functional impairments, and is also related to changes in body composition, including reduced skeletal muscle mass and increased body fat. These changes are correlated with the pathophysiology of sarcopenia, which is defined as age-related loss of skeletal muscle mass and strength. Low testosterone levels are associated with unfavorable body composition changes, and sex hormones decrease with aging. Androgen deficiency, along with lack of exercise and poor nutrition, may be among the modifiable contributors to sarcopenia. Testosterone treatment has been reported to have beneficial effects on muscle mass and function, but the results have been inconsistent. Here, we discuss the correlation between testosterone and muscle mass and function, the impact of testosterone on sarcopenia, and the probable mechanisms underlying these effects.
Aging is a global health issue. The World Health Organization predicted that the population of people over 60 years of age will reach approximately two billion by 2050. Aging is defined as a decline in functionality at the cellular, tissue, and organ levels and is related to metabolic, physiological, and functional impairments. In clinical medicine, one of the primary aging-related changes is increased body weight and waist circumference. In the elderly, low levels of physical activity can lead to an increase in body weight and body fat. Even without any weight change, body composition can change with aging [1]. Skeletal muscle mass decreases, whereas body fat, including total body, intra-abdominal, and intermuscular fat, increases [1]. This may be related to functional loss of physical activity and disability [2]. Sarcopenia is a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength, primarily associated with aging [3]. However, many conditions other than aging, such as acute and chronic diseases, immobilization, malnutrition status, and deficiency of anabolic hormones (e.g., growth hormones and sex hormones) may be involved in the etiology of sarcopenia. The correlations and causal relationships between sex hormones and muscle mass and function have not yet been fully elucidated, but both are known to decrease with age [4]. Although the results are inconsistent, epidemiological studies have found that lower testosterone levels are related to decreased muscle mass or function [567].
Here, we review the etiology of and diagnostic criteria for sarcopenia. Additionally, the relationship between sarcopenia and testosterone and the impact of testosterone treatment on muscle mass and function are also discussed.
Sarcopenia, from the Greek terms sarx for flesh and penia for loss, was originated by Rosenberg in 1989 to describe the age-related loss of muscle mass [3]. The aging process is connected to changes in body composition involving decreased muscle mass and increased body fat, with or without body weight change [1]. Muscle mass peaks in the third decade of life and decreases by approximately 1% to 2% per year [89] owing to changes in muscle fiber type and size [1011]. Muscle strength decreases by approximately 1.5% to 3.0% per year, and the rate of decline is steeper after age 50 [812]. Loss of muscle mass and function is correlated with high morbidity and mortality owing to an increased risk of frailty and falling. As these medical conditions increase social and healthcare costs, both the perceived importance of muscle mass and function and overall interest in the topic have been increasing. However, until recently, there were no clear diagnostic criteria for sarcopenia, and the detection methods and results differed according to study design. In 2010, the European Working Group on Sarcopenia in Older People (EWGSOP) proposed a practical clinical definition and consensus diagnostic criteria for age-related sarcopenia as a generalized loss of skeletal muscle mass and strength according to risk factors such as physical disability, poor quality of life, and death [13]. They suggested that sarcopenia should be diagnosed using the criteria of low muscle mass and reduced muscle function (decreased strength and/or poor physical performance) [13]; they summarized the methods for detecting muscle mass and function. They recommended estimating muscle function based on gait speed and grip strength and muscle mass according to appendicular skeletal muscle mass adjusted by height squared (Fig. 1).
Thereafter, the International Working Group on Sarcopenia (IWGS) [14], Asian Working Group for Sarcopenia (AWGS) [15], and Foundation for the National Institutes of Health (FNIH) Sarcopenia Project suggested a consensus diagnosis based on social conditions (Table 1) [16]. In general, the target population consisted of individuals aged 65 years and older, but the cutoff values varied by ethnic group or population. As a result, the specific prevalence of sarcopenia differs according to the diagnostic criteria. The large population studies have reported that sarcopenia affects over 20% of 60- to 70-year-old, and approaches 50% in those over 75 years [17]. The symptoms reported in these studies are not specific. Usually reported as a general weakness, sarcopenia can be recognized in cases of decreased muscle function, such as reduced lower leg power or functional mobility measured by short physical performance battery [17]. These symptoms can cause falls, which are related to the patient's morbidity and mortality. As a result of previous studies, and its important impact on social health, sarcopenia was admitted as a disease entity with the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) (M62.84) code in 2016. Currently, treatment of sarcopenia is a growing challenge, with many modalities being suggested and studied. Some of these include resistance exercise, consumption of protein with essential amino acids, as well as treatment with selective androgen receptor modulators, growth hormone, ghrelin agonists, myostatin antibodies and activin II receptor antagonist and testosterone [18].
Sarcopenia has numerous causes, including anorexia, inflammation, hypovitaminosis, immobilization, and hypogonadism. Testosterone, one of the representative sex hormones, is produced by Leydig cells in the testes in response to luteinizing hormone. A longitudinal study showed that testosterone levels decreased at a rate of approximately 1% per year after the age of 30 [19]; thus, approximately 40% to 70% of men older than 70 are likely to have low testosterone levels [20]. That is, along with androgen deprivation, age-related decreases in sex hormone levels can be defined as a hypogonadal state [21]. A study reported men with obesity, metabolic syndrome, and type 2 diabetes have low testosterone levels, especially when visceral adiposity is high [22]. However, in a cross-sectional study, testosterone exhibited a stepwise decrease with age with or without obesity [23], even after adjustment for other factors, such as body mass index and subscapular skinfold measurements [24]. Whether decreased serum testosterone levels explain aging-related loss of muscle mass or function has not yet been established; however, there have been several studies on the relationship between testosterone and muscle mass and function. One study reported that muscle mass was significantly associated with serum-free testosterone and insulin-like growth factor 1 (IGF-1) in relatively healthy, well-nourished elderly men [25]. Another study showed that decreases in basal blood testosterone levels in aging people may be associated with age-related declines in maximal voluntary neuromuscular performance capacity [26]. Age, arm, and leg regional fat-free mass, serum testosterone, and the free testosterone index are significantly associated with arm and leg strength in generally healthy men [25].
The exact mechanism of how androgen affects muscle has not yet been elucidated, however, a number of suggestions have been put forward. In human studies, testosterone treatment increased type I muscle fibers in both low and high concentrations, and type II muscle fibers in high concentrations [2728]. An increase in muscle fiber size is enhanced by increased protein synthesis, due to the high rate of re-utilization of intracellular amino acid by testosterone [29]. Testosterone is also reported to stimulate the mitotic activity of satellite cells in myoblast culture systems [30], which is a major source for the addition of new myonuclei into the hypertrophying muscle fibre [31]. Some studies have suggested non-genomic testosterone actions on the activation of a G-protein-linked receptor with increased intracellular Ca2+ concentration of myoblasts, which resulted in cellular growth [32], while others reported that androgens affect muscular hypertrophy by increasing IGF-1 expression [33], dependent upon Erk and mammalian target of rapamycin signaling [34].
Several randomized controlled trials (RCTs) showed the efficacy of testosterone on muscle, but the results varied by subject, dosage, and treatment methodology. These data are summarized in Table 2 [3536373839404142]. In the hypogonadal men, results were consistent regarding the effect of testosterone on muscle mass and fat mass [353637]. In the past, testosterone was given as an injection [35]. In one study, 15 hypogonadal men (mean age 68 years old, bioavailable testosterone <60 ng/dL) were randomly assigned to receive a placebo or 200 mg testosterone cypionate biweekly for 12 months. Although testosterone supplementation improved grip strength, side effects such as increased hemoglobin levels were reported. Additionally, the difficulty of administration via injection was pointed out. One of the largest studies with non-injectable testosterone was the Testosterone's Effects on Atherosclerosis Progression in Aging Men (TEAAM) trial reported in 2017 [38]. Healthy men over 60 years of age with low normal testosterone levels (total testosterone 100–400 ng/dL or free testosterone <50 pg/mL) were randomly assigned to daily treatment with 7.5 g 1% testosterone or placebo gel for 3 years. Testosterone replacement for 3 years was associated with modest improvements in chest press strength, muscle power, unloaded and loaded stair-climbing power, and lean body mass, but not leg press strength. Likewise, the results regarding the effect of testosterone on muscle function were inconsistent. Some reported that muscle power was increased [38], while others reported that there was no difference in muscle strength [3739], physical performance, activity or walking speed. These results are in line with the meta-analysis of 11 RCTs that evaluated the efficacy of androgen treatment (testosterone/5α-dihydrotestosterone) [43]. These data showed moderate efficacy in muscle strength, not only for the hypogonadal men but also in eugonadal older men or healthy young normal men who underwent supplementation of testosterone in various concentrations [41]. Both increased muscle size and decreased fat mass were seen, especially in those treated with both a supraphysiologic concentration of testosterone and exercise [42]. Additionally, it was effective in both muscle and bone when treated with calcium and vitamin D [39]. One study (not an RCT) involving muscle biopsy showed that muscle accumulation was associated with an increased fractional synthesis rate of mixed skeletal muscle proteins and that a trend existed toward a similar increase in the fractional synthesis rate of myosin heavy chain after treatment with testosterone [44]. Direct comparison is not possible between the studies, but the studies themselves indicate an important role for testosterone in developing and maintaining muscle mass and function.
Testosterone is widely used to improve impotence or increase libido but not for muscle mass or function. Its efficacy for increasing muscle mass or function varies by subject and method. In terms of the treatment of sarcopenia, sex hormones are probably not the only answer. However, it is evident that testosterone holds potential for treating sarcopenia, although the side effects, such as increased hematocrit levels and risk for cardiovascular disease, are concerning. Sarcopenia is the result of a multifactorial process, and the precise contribution of sex hormones is not evident. However, if current interest levels and research efforts continue, the role of testosterone in sarcopenia may be clarified in the near future.
References
1. Song MY, Ruts E, Kim J, Janumala I, Heymsfield S, Gallagher D. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr. 2004; 79:874–880. PMID: 15113728.

2. Harris T. Muscle mass and strength: relation to function in population studies. J Nutr. 1997; 127:1004S–1006S. PMID: 9164284.

3. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997; 127:990S–991S. PMID: 9164280.

4. Araujo AB, Travison TG, Bhasin S, Esche GR, Williams RE, Clark RV, et al. Association between testosterone and estradiol and age-related decline in physical function in a diverse sample of men. J Am Geriatr Soc. 2008; 56:2000–2008. PMID: 19016935.

5. Hyde Z, Flicker L, Almeida OP, Hankey GJ, McCaul KA, Chubb SA, et al. Low free testosterone predicts frailty in older men: the health in men study. J Clin Endocrinol Metab. 2010; 95:3165–3172. PMID: 20410223.

6. Krasnoff JB, Basaria S, Pencina MJ, Jasuja GK, Vasan RS, Ulloor J, et al. Free testosterone levels are associated with mobility limitation and physical performance in communitydwelling men: the Framingham Offspring Study. J Clin Endocrinol Metab. 2010; 95:2790–2799. PMID: 20382680.

7. Schaap LA, Pluijm SM, Deeg DJ, Penninx BW, Nicklas BJ, Lips P, et al. Low testosterone levels and decline in physical performance and muscle strength in older men: findings from two prospective cohort studies. Clin Endocrinol (Oxf). 2008; 68:42–50. PMID: 17666086.

8. Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol (1985). 2000; 88:1321–1326. PMID: 10749826.

9. Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006; 61:1059–1064. PMID: 17077199.

10. Deschenes MR. Effects of aging on muscle fibre type and size. Sports Med. 2004; 34:809–824. PMID: 15462613.

11. Evans WJ, Campbell WW. Sarcopenia and age-related changes in body composition and functional capacity. J Nutr. 1993; 123(2 Suppl):465–468. PMID: 8429405.

12. von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010; 1:129–133. PMID: 21475695.

13. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010; 39:412–423. PMID: 20392703.

14. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011; 12:249–256. PMID: 21527165.

15. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014; 15:95–101. PMID: 24461239.

16. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014; 69:547–558. PMID: 24737557.

17. Berger MJ, Doherty TJ. Sarcopenia: prevalence, mechanisms, and functional consequences. Interdiscip Top Gerontol. 2010; 37:94–114. PMID: 20703058.

18. Morley JE. Pharmacologic options for the treatment of sarcopenia. Calcif Tissue Int. 2016; 98:319–333. PMID: 26100650.

19. Morley JE, Kaiser FE, Perry HM 3rd, Patrick P, Morley PM, Stauber PM, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997; 46:410–413. PMID: 9109845.

20. Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, Gooren LJ, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males. Int J Androl. 2009; 32:1–10. PMID: 18798761.

21. Carnegie C. Diagnosis of hypogonadism: clinical assessments and laboratory tests. Rev Urol. 2004; 6(Suppl 6):S3–S8.
22. Wang C, Jackson G, Jones TH, Matsumoto AM, Nehra A, Perelman MA, et al. Low testosterone associated with obesity and the metabolic syndrome contributes to sexual dysfunction and cardiovascular disease risk in men with type 2 diabetes. Diabetes Care. 2011; 34:1669–1675. PMID: 21709300.

23. Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 1991; 73:1016–1025. PMID: 1719016.

24. Simon D, Preziosi P, Barrett-Connor E, Roger M, Saint-Paul M, Nahoul K, et al. The influence of aging on plasma sex hormones in men: the Telecom Study. Am J Epidemiol. 1992; 135:783–791. PMID: 1595678.

25. Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999; 107:123–136. PMID: 10220041.

26. Häkkinen K, Pakarinen A. Muscle strength and serum testosterone, cortisol and SHBG concentrations in middle-aged and elderly men and women. Acta Physiol Scand. 1993; 148:199–207. PMID: 8352031.
27. Kadi F. Adaptation of human skeletal muscle to training and anabolic steroids. Acta Physiol Scand Suppl. 2000; 646:1–52. PMID: 10717767.
28. Kadi F, Eriksson A, Holmner S, Thornell LE. Effects of anabolic steroids on the muscle cells of strength-trained athletes. Med Sci Sports Exerc. 1999; 31:1528–1534. PMID: 10589853.

29. Ferrando AA, Tipton KD, Doyle D, Phillips SM, Cortiella J, Wolfe RR. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol. 1998; 275:E864–E871. PMID: 9815007.
30. Powers ML, Florini JR. A direct effect of testosterone on muscle cells in tissue culture. Endocrinology. 1975; 97:1043–1047. PMID: 172315.
31. Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec. 1971; 170:421–435. PMID: 5118594.

32. Estrada M, Espinosa A, Müller M, Jaimovich E. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology. 2003; 144:3586–3597. PMID: 12865341.

33. Sculthorpe N, Solomon AM, Sinanan AC, Bouloux PM, Grace F, Lewis MP. Androgens affect myogenesis in vitro and increase local IGF-1 expression. Med Sci Sports Exerc. 2012; 44:610–615. PMID: 21946153.

34. Wu Y, Bauman WA, Blitzer RD, Cardozo C. Testosteroneinduced hypertrophy of L6 myoblasts is dependent upon Erk and mTOR. Biochem Biophys Res Commun. 2010; 400:679–683. PMID: 20816664.

35. Sih R, Morley JE, Kaiser FE, Perry HM 3rd, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997; 82:1661–1667. PMID: 9177359.

36. Srinivas-Shankar U, Roberts SA, Connolly MJ, O';Connell MD, Adams JE, Oldham JA, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010; 95:639–650. PMID: 20061435.

37. Wittert GA, Chapman IM, Haren MT, Mackintosh S, Coates P, Morley JE. Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with lownormal gonadal status. J Gerontol A Biol Sci Med Sci. 2003; 58:618–625. PMID: 12865477.

38. Storer TW, Basaria S, Traustadottir T, Harman SM, Pencina K, Li Z, et al. Effects of testosterone supplementation for 3 years on muscle performance and physical function in older men. J Clin Endocrinol Metab. 2017; 102:583–593. PMID: 27754805.
39. Kenny AM, Kleppinger A, Annis K, Rathier M, Browner B, Judge JO, et al. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J Am Geriatr Soc. 2010; 58:1134–1143. PMID: 20722847.

40. Travison TG, Basaria S, Storer TW, Jette AM, Miciek R, Farwell WR, et al. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2011; 66:1090–1099. PMID: 21697501.

41. Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005; 90:678–688. PMID: 15562020.

42. Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996; 335:1–7. PMID: 8637535.

43. Ottenbacher KJ, Ottenbacher ME, Ottenbacher AJ, Acha AA, Ostir GV. Androgen treatment and muscle strength in elderly men: a meta-analysis. J Am Geriatr Soc. 2006; 54:1666–1673. PMID: 17087692.

44. Brodsky IG, Balagopal P, Nair KS. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men: a clinical research center study. J Clin Endocrinol Metab. 1996; 81:3469–3475. PMID: 8855787.
Fig. 1
The European Working Group on Sarcopenia in Older People (EWGSOP) suggested algorithm for sarcopenia case ascertainment in older individuals [13]. Data from Cruz-Jentoft et al (Age Ageing 2010;39:412-23) [13] with original copyright holder's permission.
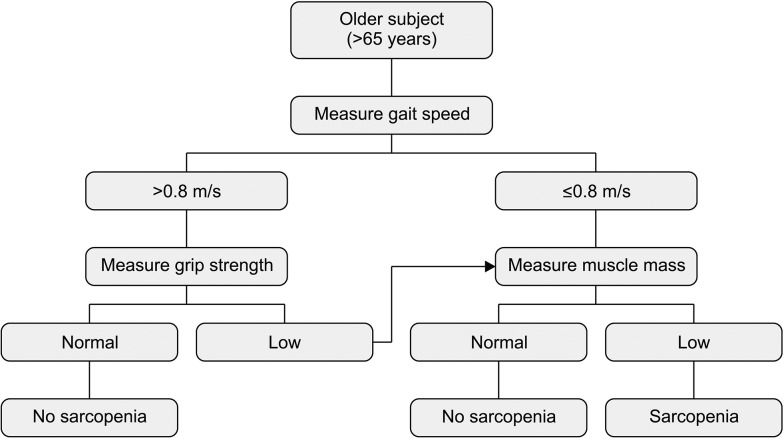
Table 1
Diagnostic criteria according to different study groups
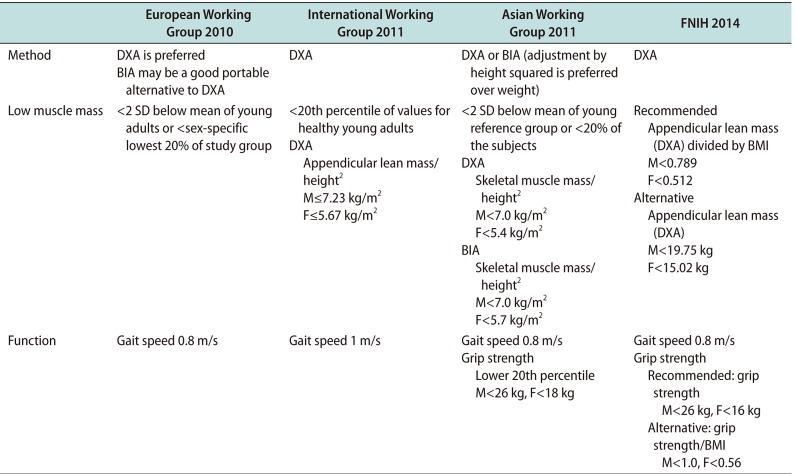
Table 2
Randomized controlled trial intervention studies addressing the effects of T on muscle strength and physical function in older patients
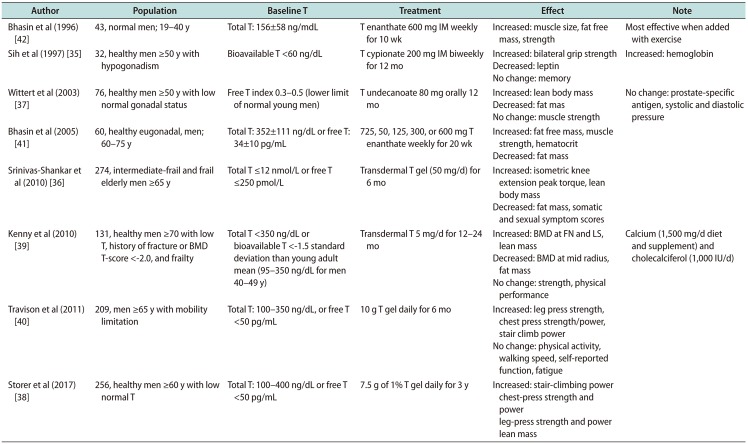
| Author | Population | Baseline T | Treatment | Effect | Note |
|---|---|---|---|---|---|
| Bhasin et al (1996) [42] | 43, normal men; 19–40 y | Total T: 156±58 ng/mdL | T enanthate 600 mg IM weekly for 10 wk | Increased: muscle size, fat free mass, strength | Most effective when added with exercise |
| Sih et al (1997) [35] | 32, healthy men ≥50 y with hypogonadism | Bioavailable T <60 ng/dL | T cypionate 200 mg IM biweekly for 12 mo | Increased: bilateral grip strength | Increased: hemoglobin |
| Decreased: leptin | |||||
| No change: memory | |||||
| Wittert et al (2003) [37] | 76, healthy men ≥50 y with low normal gonadal status | Free T index 0.3–0.5 (lower limit of normal young men) | T undecanoate 80 mg orally 12 mo | Increased: lean body mass | No change: prostate-specific antigen, systolic and diastolic pressure |
| Decreased: fat mas | |||||
| No change: muscle strength | |||||
| Bhasin et al (2005) [41] | 60, healthy eugonadal, men; 60–75 y | Total T: 352±111 ng/dL or free T: 34±10 pg/mL | 725, 50, 125, 300, or 600 mg T enanthate weekly for 20 wk | Increased: fat free mass, muscle strength, hematocrit | |
| Decreased: fat mass | |||||
| Srinivas-Shankar et al (2010) [36] | 274, intermediate-frail and frail elderly men ≥65 y | Total T ≤12 nmol/L or free T ≤250 pmol/L | Transdermal T gel (50 mg/d) for 6 mo | Increased: isometric knee extension peak torque, lean body mass | |
| Decreased: fat mass, somatic and sexual symptom scores | |||||
| Kenny et al (2010) [39] | 131, healthy men ≥70 with low T, history of fracture or BMD T-score <−2.0, and frailty | Total T <350 ng/dL or bioavailable T <−1.5 standard deviation than young adult mean (95–350 ng/dL for men 40–49 y) | Transdermal T 5 mg/d for 12–24 mo | Increased: BMD at FN and LS, lean mass | Calcium (1,500 mg/d diet and supplement) and cholecalciferol (1,000 IU/d) |
| Decreased: BMD at mid radius, fat mass | |||||
| No change: strength, physical performance | |||||
| Travison et al (2011) [40] | 209, men ≥65 y with mobility limitation | Total T: 100–350 ng/dL, or free T <50 pg/mL | 10 g T gel daily for 6 mo | Increased: leg press strength, chest press strength/power, stair climb power | |
| No change: physical activity, walking speed, self-reported function, fatigue | |||||
| Storer et al (2017) [38] | 256, healthy men ≥60 y with low normal T | Total T: 100–400 ng/dL or free T <50 pg/mL | 7.5 g of 1% T gel daily for 3 y | Increased: stair-climbing power chest-press strength and power leg-press strength and power lean mass |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download