Abstract
Purpose
This study investigated how adding Korean red ginseng extract (KRG) to folinic acid, fluorouracil and oxaliplatin (FOLFOX) chemotherapy affected the rate of splenomegaly in colon cancer.
Methods
This retrospective study analyzed 42 patients who were randomly assigned to receive a FOLFOX regimen with or without KRG. Spleen volume change was assessed by computed tomography scans measured before surgery (presurgery volume) and 3 weeks after cessation of the 12th cycle of FOLFOX (postchemotherapy volume).
Results
All patients showed increased spleen volume. No difference was observed in median presurgery and postchemotherapy volume between the KRG and control groups. However, a ratio defined as postchemotherapy volume divided by presurgery volume was significantly lower in the KRG group than the control group (median, 1.38 [range, 1.0–2.8] in KRG group vs. median, 1.89 [range, 1.1–3.0] in control group, P = 0.028). When splenomegaly was defined as a >61% increase in spleen volume, the rate of splenomegaly was significantly lower in the KRG group than the control group (28.6% vs. 61.9%, P = 0.03). KRG consumption was inversely associated with developing splenomegaly in multivariate analysis.
Colon and rectal cancer are the third most common malignancies worldwide [1], and the third highest cancer by incidence in Korea [2]. Although surgery remains one of the most important treatment modalities, adjuvant chemotherapy after resection is recommended to reduce local and systemic recurrence in colon cancer.
Folinic acid, fluorouracil and oxaliplatin (FOLFOX) is a chemotherapy regimen for colorectal cancer, made up of three drugs including folinic acid (leucovorin), fluorouracil (5-FU) and oxaliplatin. Because adding oxaliplatin to a 5-FU and leucovorin regimen or capecitabine is suggested to reduce the probability of recurrence upto 20%, FOLFOX is regarded as a standard adjuvant chemotherapy treatment for stage III colon cancer [3456]. However, major serious adverse reactions such as anaphylaxis and allergic reaction, neuropathy, pulmonary toxicities, hepatotoxicity, cardiovascular, rhabdomyolysis, as well as minor side effects are reported during oxaliplatin-based chemotherapy. These toxicities have an impact on patient quality of life and sometimes restrict applications of the FOLFOX regimen.
Sinusoidal injury of the liver is a known ox aliplatin-specific complication. The histopathological features of sinusoidal injury include sinusoidal dilatation and congestion, small vessel obliteration, hepatocyte plate disruption, parenchymal extinction lesions, nodular regenerative hyperplasia, peliosis, and veno-occlusive disease [7]. Although the direct reasons for sinusoidal injury after oxaliplatin-based chemotherapy are not well understood, initial toxic effects to sinusoidal endothelial cells may cause sinusoidal wall disruption, eventually leading to fibrotic change with sinusoidal matrix deposition [8]. A report found that 78% of patients showed sinusoidal obstruction, complicated by perisinusoidal fibrosis and veno-occlusive lesion after use of oxaliplatin as a preoperative chemotherapy regimen [8]. The rate of this kind of liver damage is relatively higher after use of oxaliplatin than with other kinds of chemotherapy agents such as Irinotecan or 5-FU/leucovorin [910].
Overman et al. [11] reported that increased spleen size correlates with cumulative oxaliplatin dose. In addition, splenomegaly could be used as a simple biomarker to predict oxaliplatin-induced sinusoidal liver injury [11]. Other studies demonstrate that oxaliplatin use can cause splenomegaly in colorectal cancer patients [121314]. Clinically, splenic enlargement might induce thrombocytopenia after long-term follow-up [1113].
Robinson et al. [15] reported that FOLFOX injection via an intraperitoneal route induces sinusoidal dilatation and hepatocyte atrophy in the liver of a murine model, causing sinusoidal obstruction syndrome (SOS). The study showed that dietary supplementation with the antioxidant butylated hydroxyanisole prevents SOS.
Korean red ginseng is a heat-and-steam processed product of Korean ginseng (Panax ginseng Meyer). Korean red ginseng has a variety of pharmacological activities including anti-inflammatory and antitumor effects. Studies demonstrate that Korean red ginseng attenuates ethanol-induced oxidative injury of the liver in a murine model [1617].
Based on these results, we hypothesized that Korean red ginseng might protect against oxaliplatin-induced liver damage. Considering the correlation between splenomegaly and sinusoidal liver injury after oxaliplatin-based chemotherapy, whether Korean red ginseng extract (KRG) offers benefits for reducing development of splenomegaly in colon cancer patients who receive adjuvant oxaliplatin-based chemotherapy remains unclear. Thus, this study investigated whether adding KRG to FOLFOX affected the rate of splenomegaly in colon cancer patients.
This retrospective study analyzed 42 patients who underwent surgery for adenocarcinoma of the colon and received FOLFOX adjuvant chemotherapy between June 2012 and June 2016. The patients were part of a cohort that was prospectively designed to evaluate the clinical efficacy of Korean red ginseng for breast, pancreas and colon cancer after adjuvant chemotherapy. Twenty-one patients were allocated into each of the 2 groups: the Korean red ginseng group and the control group. This study was a retrospective study and a subgroup analysis using prospectively collected data. This study was approved by Gangnam Severance Hospital, Yonsei University College of Medicine Institutional Review Board (3-2016-0287). Informed consent was waived for this retrospective study.
All patients received 12 cycles of FOLFOX regimen. For stage III colon cancer, adjuvant FOLFOX chemotherapy is a standard chemotherapy. Patients with stage II colon cancer with high risk factors such as T4, obstruction or perforation in clinical presentation, lymphovascular invasion, retrieval of fewer than 12 lymph nodes, or poorly differentiated histology were also treated with FOLFOX regimen.
The dose schedule given every 2 weeks was oxaliplatin (85 mg/m2 IV infusion of more than 2 hours on day 1), leucovorin (400 mg/m2 IV infusion of more than 2 hours on day 1), and 5-FU (400 mg/m2 IV bolus injection on day 1, followed by 2,400 mg/m2 continuous IV injection over 46 hours). A total of 12 cycles was planned. Antiemetic prophylaxis with 5-HT3-receptor antagonist was given every cycle. Patients in the Korean red ginseng group received a total 3 g of KRG per day during the chemotherapy periods. Safety of 3 g KRG intake in a day was already proved in a previous randomized controlled trial on the effect of improving blood circulation [18]. In addition, another prospective randomized trial investigating the immune effect of KRG in patients with colon cancer who had a curative resection and chemotherapy used daily 3 g of KRG [19]. Based on these previous results, the initial prospective study chose to use 3 g of KRG as a daily dose.
Spleen volume was measured by hand-tracing the organ outline on CT images and calculating using commercially available volume-measuring software (Voxel Plus 2.0; Medisys Co., Daejeon, Korea) (Fig. 1). The spleen outline on each abdominal axial image and the sum of the marked area, accounting for slice thickness, was used. Spleen volume was measured by an investigator who was blinded to patient clinical data. Spleen volume was assessed twice using CT images performed before index surgical resection (presurgical volume) and 3 weeks after cessation of the 12 FOLFOX regimen treatments (postchemotherapy volume). The spleen volume ratio was defined as postchemotherapy volume divided by presurgery volume. Splenomegaly was defined as a >61% increase in spleen volume compared with initial measurements.
Differences in clinicopathologic features and volumetric changes were analyzed using 2-sided Pearson chi-square test or Fisher exact test for categorical variables and a Student t-test for continuous variables. To define splenomegaly, cut off values of the ratio providing the best separation between thrombocytopenia group and normal groups after chemotherapy cessation were obtained using receiver operating characteristic (ROC) curve analyses (Fig. 2). Exact logistic regression for multivariate analysis of factors associated with splenomegaly was performed. A P-value < 0.05 was considered to indicate statistical significance. All calculations and analysis were performed using the IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA).
Patient characteristics for the 2 groups are in Table 1. No significant difference was observed in sex, age, body mass index, body surface area (BSA), pathologic stage or tumor location.
Median spleen volume at initial stage before surgery (presurgical volume) was 132 cm3 (range, 45–388 cm3) in the Korean red ginseng group and 141 cm3 (range, 64–250 cm3) in the control group (P = 0.753). All patients in this study underwent abdominopelvic computed tomography 3 weeks after completion of 12 cycles of FOLFOX chemotherapy. The median spleen volume after chemotherapy (postchemotherapy volume) was 212 cm3 (range, 95–533 cm3) in the Korean red ginseng group and 247 cm3 (range, 84–564 cm3), in the control group (P = 0.122). All patients showed increased spleen volume after completion of chemotherapy. The median spleen volume ratio was 1.38 (range, 1.03–2.81) in the Korean red ginseng group and 1.89 (range, 1.13–3.01; P = 0.028) in the control group.
According to ROC curve for correlation of occurrence of thrombocytopenia with spleen volume ratio, 1.61 was set as the cutoff value based on Youden index. Thus, we defined splenomegaly as a >61% increase in spleen volume compared with initial measurements.
The rate of splenomegaly was significantly lower in the Korean red ginseng group than the control group (28.6% Korean red ginseng group vs. 61.9% control group, P = 0.03) (Table 2).
In the Korean red ginseng and control groups, mean values for AST, total bilirubin and alkaline phosphatase were significantly increased and platelet count was significantly reduced after 12 cycles of chemotherapy. ALT increased significantly after chemotherapy in the control group, but did not significantly increase in the Korean red ginseng group (Table 3).
No difference was seen in univariate analysis in the rate of splenomegaly according to sex, age, body mass index, BSA, tumor location, or pathology stage. Thrombocytopenia was significantly associated with splenomegaly in univariate analysis. In multivariate analysis, consumption of Korean red ginseng was associated with reduced incidence of splenomegaly (Table 4).
There was no difference of rate of more than grade 3 neutropenia between the 2 groups (57.1% in the Korean red ginseng group vs. 38.1% in the control group, P = 0.354). No difference was seen on the number of patients who used granulocyte colony stimulating factors during the chemotherapy periods between the 2 groups. There was only 1 patient in the control group who showed more than grade 3 elevated AST or ALT (Table 5).
This study demonstrated that adding KRG during chemotherapy periods reduced oxaliplatin-mediated splenomegaly in colon cancer patients. These outcomes implied that Korean red ginseng might protect against sinusoidal injury of the liver induced by FOLFOX chemotherapy, although the basic pathophysiology should be evaluated.
In managing colorectal cancer with liver metastasis, preoperative chemotherapy before resection reduces metastasis size, thus enhancing the likelihood of curative resection [20]. However, sinusoidal injury of the liver is a well-known adverse impact of oxaliplatin, first described by Rubbia-Brandt et al. [8] in 2004. Sinusoidal dilatation is significantly correlated with an increasing number of oxaliplatin-based chemotherapy cycles [7]. Oxaliplatin-induced liver injury (also called sinusoidal obstruction syndrome) is related to increased morbidity [2122].
According to our results, Korean red ginseng consumption was associated with reduced incidence of splenomegaly after FOLFOX chemotherapy. However, the mechanism underlying protection by Korean red ginseng against oxaliplatin-mediated splenomegaly remains unknown. A recent experimental study in a murine model demonstrated molecular pathways affecting the progression of SOS by FOLFOX treatment [15]. Endothelial damage by administration of a FOLFOX regimen provokes a prothrombotic state within the liver with upregulation of plasminogen activator inhibitor-1 (PAI-1), von Willebrand factor and Factor X. In addition, the study also suggests that metallothionein 1, heme oxygenase 1, and superoxide dismutase 3, which are genes implicated in the oxidative stress, are upregulated. An antioxidant diet using butylated hydroxyanisole supplementation, which is associated with significantly increased NQO1 expression, reduces severity of sinusoidal liver injury [15]. KRG have antioxidant effects in vitro and in vivo in human studies [2324]. In another study, Ki et al. [25] reported that KRG inhibits hepatocyte degeneration and collagen accumulation by blocking TGFb1 and PAI-1 expression. All these mechanisms might have positive effects in protection against sinusoidal liver injury. Nevertheless, further studies are required to clarify the fundamental protection by Korean red ginseng against oxaliplatin-based splenomegaly or ability to reduce liver toxicity.
Many studies investigated the association of oxaliplatin-based chemotherapy with splenomegaly, but did not use the same definition of splenomegaly. Angitapalli et al. [12] used splenic index (SI), defined as “splenic length (maximum longitudinal dimension) × splenic width (transversely across the hilum) × splenic height (cephalocaudal dimension)” measured by computed tomography. They defined a significant increase in SI as more than 50% increase compared to baseline. Several other studies used CT-based volumetry program and defined splenomegaly as more than 50% increase from baseline volume [111314]. In contrast, Imai et al. [26] reported that splenic volume enlargement more than 25% measured by CT-based volumetry was the only independent predictor of SOS. Other clinical factors such as accumulative chemotherapy cycle, thrombocytopenia, and interval between completion of chemotherapy and surgery were not significant factors in multivariate analysis. Our study defined splenomegaly as more than 61% increase in splenic volume compared to baseline volume. Although this definition is an arbitrary cutoff, the value was the most discriminating point by ROC curve for differentiating thrombocytopenia events after cessation of chemotherapy among our patients. However, the definition of splenomegaly should be investigated in a large cohort study.
This study has some limitations. The relatively small number of patients and lack of sample size calculation were the main weak points. However, as far as we know, the impact of Korean red ginseng on protection against oxaliplatin-induced splenomegaly has rarely been investigated. Thus, accurately measuring the proper sample size for this clinical study was practically impossible.
In conclusion, the addition of KRG to FOLFOX chemotherapy could protect against oxaliplatin-mediated splenomegaly in colon cancer patients. The fundamental pathway should be evaluated in further studies.
Figures and Tables
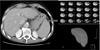 | Fig. 1CT based voulmetry. Spleen volume was measured by manual calculation for CT slices and summation of boundaries was determined by a 3-dimensional program. |
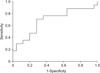 | Fig. 2Receiver operating characteristic (ROC) curve. ROC curve revealed that the most accurate cutoff value predicting occurrence of thrombocytopenia after cessation of chemotherapy was Youden's index 1.612 (area under the curve, 0.687; sensitivity, 70.6%; specificity, 28%). |
ACKNOWLEDGMENTS
This research was supported by a research grant from the Korea Ginseng Corporation. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
This article was presented at Joint Congress of the 6th Biennial Congress of the Asian-Pacific Hepato-Pancreato-Biliary Association & the 29th Meeting of Japanese Society of Hepato-Biliary-Pancreatic Surgery, Yokohama, Japan, Jun 7-10, 2017.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.

2. Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015; 47:127–141.

3. André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004; 350:2343–2351.

4. Yothers G, O'Connell MJ, Allegra CJ, Kuebler JP, Colangelo LH, Petrelli NJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011; 29:3768–3774.
5. Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011; 29:1465–1471.

6. Jeong WK, Shin JW, Baek SK. Oncologic outcomes of early adjuvant chemotherapy initiation in patients with stage III colon cancer. Ann Surg Treat Res. 2015; 89:124–130.

7. Nam SJ, Cho JY, Lee HS, Choe G, Jang JJ, Yoon YS, et al. Chemotherapy-associated hepatopathy in korean colorectal cancer liver metastasis patients: oxaliplatin-based chemotherapy and sinusoidal injury. Korean J Pathol. 2012; 46:22–29.

8. Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004; 15:460–466.

9. Mehta NN, Ravikumar R, Coldham CA, Buckels JA, Hubscher SG, Bramhall SR, et al. Effect of preoperative chemotherapy on liver resection for colorectal liver metastases. Eur J Surg Oncol. 2008; 34:782–786.

10. Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006; 24:2065–2072.

11. Overman MJ, Maru DM, Charnsangavej C, Loyer EM, Wang H, Pathak P, et al. Oxaliplatin-mediated increase in spleen size as a biomarker for the development of hepatic sinusoidal injury. J Clin Oncol. 2010; 28:2549–2555.

12. Angitapalli R, Litwin AM, Kumar PR, Nasser E, Lombardo J, Mashtare T, et al. Adjuvant FOLFOX chemotherapy and splenomegaly in patients with stages II-III colorectal cancer. Oncology. 2009; 76:363–368.

13. Jung EJ, Ryu CG, Kim G, Kim SR, Park HS, Kim YJ, et al. Splenomegaly during oxaliplatin-based chemotherapy for colorectal carcinoma. Anticancer Res. 2012; 32:3357–3362.
14. Kim MJ, Han SW, Lee DW, Cha Y, Lee KH, Kim TY, et al. Splenomegaly and its associations with genetic polymorphisms and treatment outcome in colorectal cancer patients treated with adjuvant FOLFOX. Cancer Res Treat. 2016; 48:990–997.

15. Robinson SM, Mann J, Vasilaki A, Mathers J, Burt AD, Oakley F, et al. Pathogenesis of FOLFOX induced sinusoidal obstruction syndrome in a murine chemotherapy model. J Hepatol. 2013; 59:318–326.

16. Park HM, Kim SJ, Mun AR, Go HK, Kim GB, Kim SZ, et al. Korean red ginseng and its primary ginsenosides inhibit ethanol-induced oxidative injury by suppression of the MAPK pathway in TIB-73 cells. J Ethnopharmacol. 2012; 141:1071–1076.

17. Han JY, Lee S, Yang JH, Kim S, Sim J, Kim MG, et al. Korean red ginseng attenuates ethanol-induced steatosis and oxidative stress via AMPK/Sirt1 activation. J Ginseng Res. 2015; 39:105–115.

18. Shin KS, Lee JJ, Kim YI, Yu JY, Park ES, Im JH, et al. Effect of Korean red ginseng extract on blood circulation in healthy volunteers: a randomized, double-blind, placebo-controlled trial. J Ginseng Res. 2007; 31:109–116.
19. Boo YJ, Park JM, Kim J, Suh SO. Prospective study for Korean red ginseng extract as an immune modulator following a curative surgery in patients with advanced colon cancer. J Ginseng Res. 2007; 31:54–59.
20. Masi G, Loupakis F, Pollina L, Vasile E, Cupini S, Ricci S, et al. Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Ann Surg. 2009; 249:420–425.

21. Nakano H, Oussoultzoglou E, Rosso E, Casnedi S, Chenard-Neu MP, Dufour P, et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg. 2008; 247:118–124.

22. Soubrane O, Brouquet A, Zalinski S, Terris B, Brézault C, Mallet V, et al. Predicting high grade lesions of sinusoidal obstruction syndrome related to oxaliplatin-based chemotherapy for colorectal liver metastases: correlation with post-hepatectomy outcome. Ann Surg. 2010; 251:454–460.
23. Yuan HD, Kim JT, Kim SH, Chung SH. Ginseng and diabetes: the evidences from in vitro, animal and human studies. J Ginseng Res. 2012; 36:27–39.

24. Seo SJ, Cho JY, Jeong YH, Choi YS. Effect of Korean red ginseng extract on liver damage induced by short-term and long-term ethanol treatment in rats. J Ginseng Res. 2013; 37:194–200.





 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download